
Studies find that microbiome changes may be a signature for ME/CFS
Wednesday February 8 2023ME/CFS is a serious chronic and debilitating disease characterized by a range of symptoms including fatigue post-exertional malaise sleep disturbance cognitive difficulties pain and gastrointestinal issues. The causes of the disease are unknown and there are no treatments.In one study senior author Brent L. Williams Ph.D. assistant professor W. Ian Lipkin M.D. John Snow Professor of Epidemiology and director of the Center for Infection and Immunity at the Columbia University Mailman School of Public Health in New York City and their collaborators analyzed the genetic makeup of gut bacteria in fecal samples collected from a geographically diverse cohort of 106 people with ME/CFS and 91 healthy controls. The results revealed key differences in microbiome diversity quantity metabolic pathways and interactions between species of gut bacteria.Dr. Williams and his colleagues found that people with ME/CFS had abnormally low levels of several bacterial species compared to healthy controls including Faecalibacterium prausnitzii (F. prausnitzii) and Eubacterium rectale. These health-promoting bacteria produce a short chain fatty acid called butyrate a bacterial metabolite or by-product that plays an important role in maintaining gut health. An acetate-producing bacterium was also reduced in samples obtained from people with ME/CFS.More detailed metabolomic analyses confirmed that a reduction in these bacteria was associated with reduced butyrate production in ME/CFS. Butyrate is the primary energy source for cells that line the gut providing up to 70% of their energy requirements support for the gut immune system and protection against diseases of the digestive tract. Butyrate tryptophan and other metabolites detected in the blood are important for regulating immune metabolic and endocrine functions.While species of butyrate-producing bacteria decreased there were increased levels of nine other species in ME/CFS including Enterocloster bolteae and Ruminococcus gnavus which are associated with autoimmune diseases and inflammatory bowel disease respectively.Dr. Williams group also reported that an abundance of F. prausnitzii was inversely associated with fatigue severity in ME/CFS suggesting a possible link between gut bacteria and disease symptoms. More research is needed to determine if differences in the gut microbiome are a consequence or cause of symptoms.The findings indicate that imbalances in these 12 species of bacteria could be used as biomarkers for ME/CFS classification potentially providing consistent measurable targets to improve diagnosis.The gut microbiome is an ecosystem with complex interactions between bacteria where microbes can exchange or compete for nutrients metabolites or other molecular signals. Researchers found notable differences in the network of species interactions in people with ME/CFS—including unique interactions between F. prausnitzii and other species. This indicates that there is an extensive rewiring of bacterial networks in ME/CFS.“In addition to differences in individual species in ME/CFS focusing a lens on community interaction dynamics may add greater specificity to the broad definition of dysbiosis distinguishing between other diseases in which the gut microbiome becomes imbalanced ” said Dr. Williams. “This is also important for generating new testable hypotheses about the underlying mechanisms and mediators of dysbiosis in ME/CFS and may eventually inform strategies to correct these imbalances.”A balanced microbiome is also essential for a variety of neural systems especially immune regulation and coupling between energy metabolism and blood supply in the brain as well as the function of the nerves that supply the gut.In another study at the Jackson Laboratory in Farmington Connecticut Julia Oh Ph.D. associate professor and Derya Unutmaz M.D. professor teamed up with other ME/CFS experts to study microbiome abnormalities in different phases of ME/CFS. Dr. Ohs team collected and analyzed clinical data fecal samples and blood samples from 149 people with ME/CFS who had been diagnosed within the previous four years (74 short-term) or who had been diagnosed more than 10 years ago (75 long-term) and 79 healthy controls.The results showed that the short-term group had less microbial diversity while the long-term group established a stable but individualized gut microbiome similar to healthy controls. Dr. Oh and her colleagues found lower levels of several butyrate-producing species including F. prausnitzii especially in the short-term participants. There was also a reduction in species associated with tryptophan metabolism in all ME/CFS participants compared to controls.Dr. Ohs group also collected detailed clinical and lifestyle data from participants. By combining these data with genetic and metabolome data the team developed a way to accurately classify and differentiate ME/CFS from healthy controls. Using this approach they found that individuals with long-term ME/CFS had a more balanced microbiome but showed more severe clinical symptoms and progressive metabolic irregularities compared to the other groups.Both studies identify potential biomarkers for ME/CFS which may inform diagnostic tests and disease classification. Understanding the connection between disturbances in the gut microbiome and ME/CFS may also guide the development of new therapeutics.Additional research is required to learn more about the pathophysiological implications of butyrate and other metabolite deficiencies in ME/CFS. Future studies will determine how gut microbe disturbances contribute to symptoms including changes during disease progression.NINDS is the nations leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.Guo et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host & Microbe February 8 2023. DOI 10.1016/j.chom.2023.01.004.Xiong et al. Multi-omics of host-microbiome interactions in short- and long-term Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Cell Host & Microbe February 8 2023. DOI 10.1016/j.chom.2023.01.001.Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Research NetworkU.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/studies-find-microbiome-changes-may-be-signature-mecfs

Women treated for breast cancer may age faster than cancer-free women
Wednesday July 19 2023“Of the three treatment classes we looked at radiation therapy had the strongest associations with the biologic age measures assessed in the study ” noted Jack Taylor M.D. Ph.D. the senior author on the paper who is an Emeritus Scientist at NIEHS. “The increases can be detected years after treatment.”The researchers used three different established “methylation clocks” to determine if there were changes in a womens biological age between the two time points. The clocks measure naturally occurring chemical modifications to a persons DNA known as methylation changes. Small variations in methylation patterns can help determine a persons risk of developing an age-related disease.Women diagnosed with breast cancer had faster aging rates by all three clocks with no significant racial differences when compared to women who did not develop breast cancer.Next the scientists examined whether biological age was associated with specific treatment regimens such as surgery chemotherapy radiation therapy and endocrine therapy. Among women with breast cancer aging rates varied by treatment type.“Radiation is a valuable treatment option for breast cancer and we dont yet know why it was most strongly associated with biological age ” noted Dale Sandler Ph.D. chief of the NIEHS Epidemiology Branch and a co-author on the paper. “This finding supports efforts to minimize radiation exposures when possible and to find ways to mitigate adverse health effects among the approximately 4 million breast cancer survivors living in the United States.”The scientists emphasized that women should not abandon radiation therapy as an option based on this research. Current breast cancer treatments that include radiation are very effective in preventing breast cancer from spreading.“Women faced with a breast cancer diagnosis should discuss all possible treatment options with their doctors to determine the best course of treatment for them ” said Katie OBrien Ph.D. a scientist in the NIEHS Epidemiology Branch and a co-author on the paper.The lead author Jacob Kresovich Ph.D. is currently a researcher in the Cancer Epidemiology Program at the Moffit Cancer Center. He began this work while a post-doctoral researcher in Taylors research group in the intramural research program at NIEHS. The study was published in the Journal of the National Cancer Institute.Grant Numbers Z01-ES049033 Z01-ES049032 Z01-ES044005Kresovich JK OBrien KM Xu Z Weinberg CR Sandler DP Taylor JA. 2023. Changes in methylation-based aging in women who do and do not develop breast cancer. Journal of the National Cancer Institute. https //doi.org/10.1093/jnci/djad117U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/women-treated-breast-cancer-may-age-faster-cancer-free-women

Overdose deaths involving buprenorphine did not proportionally increase with new flexibilities in prescribing
Friday January 20 2023These data are consistent with a recent study reporting that COVID-era expansion of methadone access for the treatment of opioid use disorder was not associated with an increase in methadone-involved overdose deaths.In 2021 nearly 107 000 people died of a drug overdose with 75% of those deaths involving an opioid. The overall rise in overdose deaths is largely attributable to the proliferation in the drug supply of illicit fentanyl a highly potent synthetic opioid. Though the benefits of providing medication for opioid use disorder are well-known only 22% of people with opioid use disorder receive medications. Buprenorphine one of these medications helps reduce opioid misuse decrease risk for injection-related infectious diseases and decrease risk for fatal and non-fatal overdoses.“Research has shown beyond a doubt that medications for opioid use disorder are overwhelmingly beneficial and can be lifesaving yet they continue to be vastly underused ” said NIDA Director and senior author Nora Volkow M.D. “Expanding more equitable access to these medications for people with substance use disorders is a critical part of our nations response to the overdose crisis. The findings from this study strengthen existing evidence suggesting that greater flexibility in prescribing may be one safe method for working toward this goal.”While the recently signed Fiscal Year 2023 omnibus appropriations bill amended the Controlled Substances Act to eliminate the requirement that clinicians obtain a specific waiver to prescribe buprenorphine to treat opioid use disorder buprenorphine remains a Schedule III controlled substance with restrictions on prescribing. During the onset of the COVID-19 pandemic the United States government implemented prescribing flexibilities to facilitate buprenorphine access for patients with opioid use disorder. These updated policies allowed clinicians to remotely prescribe buprenorphine to new patients without conducting in-person examinations expanded payment for telehealth services and provided flexibility on accepted communication technologies to deliver clinical care for people with substance use disorders via telehealth.To investigate the impact of these policy changes researchers used data from the CDCs State Unintentional Drug Overdose Reporting System (SUDORS) to assess overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia. SUDORS combines data from death certificates medical examiner and coroner reports and postmortem toxicology testing.Researchers found that buprenorphine was involved in a very small proportion of drug overdose deaths between July 2019 and June 2021. During this study period there were 1 955 buprenorphine-involved overdose deaths which represented 2.2% of the 89 111 total overdose deaths and 2.6% of the 74 474 opioid-involved overdose deaths recorded in the SUDORS dataset. Between April 2020 and June 2021 when buprenorphine prescribing regulations were relaxed in response to the COVID-19 pandemic the researchers found that while monthly opioid-involved overdose deaths increased overall the proportion of those deaths involving buprenorphine did not increase.Additionally the study found that 92.7% of buprenorphine-involved overdose deaths also involved at least one other drug compared to 67.2% of deaths involving an opioid other than buprenorphine. Specifically compared with other opioid-involved overdose deaths buprenorphine-involved overdose deaths were more likely to also involve prescription medications such as benzodiazepines (36.9% vs. 14.5%) antidepressants (13.9% vs. 5.0%) and anticonvulsants (18.6% vs. 5.4%). Buprenorphine-involved overdose deaths were less likely to also“These findings help us better understand the circumstances of overdose deaths involving buprenorphine which is crucial in our ability to inform policy ensure safety and improve clinical outcomes for people with substance use disorders ” said Lauren Tanz Sc.D. an epidemiologist at CDCs National Center for Injury Prevention and Control and lead author on the study. “It is important to note the presence of other drugs in overdose deaths involving buprenorphine. The complex nature of substance use disorders and polysubstance use requires specific strategies to address it.”Data also showed that non-Hispanic white people represented a higher proportion of the deaths involving buprenorphine (86.1%) compared to deaths related to other opioids (69.4%). In contrast buprenorphine-involved overdose deaths included fewer Black non-Hispanic people (5.7%) and Hispanic people (5.5%) compared with other opioid-involved overdose deaths (18.8% and 9.4% respectively) which the authors note might be related to inequitable access to treatment.Regardless of the drugs involved the investigators found that most people who died of an overdose involving any opioid including buprenorphine had no evidence of current treatment for substance use disorders. In addition most deaths occurred without another person being present a known risk factor for fatal overdose.For more information on substance and mental health treatment programs in your area call the free and confidential National Helpline 1-800-662-HELP (4357) or visit www.FindTreatment.gov.LJ Tanz et al. Trends and Characteristics of Buprenorphine-Involved Overdose Deaths Prior to and During the COVID-19 Pandemic. JAMA Network Open. DOI 10.1001/jamanetworkopen.2022.51856 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/overdose-deaths-involving-buprenorphine-did-not-proportionally-increase-new-flexibilities-prescribing

Probiotic markedly reduces S. aureus colonization in Phase 2 trial
Tuesday January 17 2023Methicillin-resistant S. aureus or MRSA is familiar to many people as a cause of serious disease. Less well known is that S. aureus often lives in the nose on the body and in the gut without causing any harm. However if the skin barrier is broken or the immune system compromised these colonizing bacteria can cause serious skin bone lung and blood infections.The prevention of S. aureus infections using approaches to “decolonize” the body has gained increased attention as the spread of antibiotic resistance limits treatment options. Some decolonization strategies are controversial because they also require large amounts of antibiotics raising concerns about damage to the microbiota and the development of antibiotic resistance. So far it appears that only nasal S. aureus colonization can be targeted with topical antibiotics without doing too much harm but bacteria quickly can recolonize in the nose from the gut.Probiotics digestive supplements containing live microorganisms may be a way to complement or replace antibiotics. Probiotic Bacillus is especially promising because it is administered orally as spores that can survive passage through the stomach and then temporarily grow in the intestine. In prior studies Dr. Ottos group discovered an S. aureus sensing system needed for S. aureus to grow in the gut. They also found that fengycins Bacillus lipopeptides that are part peptide and part lipid prevent the S. aureus sensing system from functioning thereby eliminating the bacteria.In the clinical trial conducted in Thailand the research team tested whether this approach works in people. They enrolled 115 healthy participants all of whom were colonized naturally with S. aureus. A group of 55 people received B. subtilis probiotic once daily for four weeks; a control group of 60 people received a placebo. After four weeks researchers evaluated the participants S. aureus levels in the gut and nose. They found no changes in the control group but in the probiotic group they observed a 96.8% S. aureus reduction in the stool and a 65.4% reduction in the nose.“The probiotic we use does not kill S. aureus but it specifically and strongly diminishes its capacity to colonize ” Dr. Otto said. “We think we can target the bad S. aureus while leaving the composition of the microbiota intact.”The researchers also found that levels of S. aureus bacteria in the gut far exceeded S. aureus in the nose which for decades has been the focus of staph infection prevention research. This finding adds to the potential importance of S. aureus reduction in the gut.“Intestinal S. aureus colonization has been evident for decades but mostly neglected by researchers because it was not a viable target for antibiotics ” Dr. Otto said. “Our results suggest a way to safely and effectively reduce the total number of colonizing S. aureus and also call for a categorical rethinking of what we learned in textbooks about S. aureus colonization of the human body.”The researchers plan to continue their work by testing the probiotic in a larger and longer trial. They note that their approach probably does not work as quickly as antibiotics but can be used for long periods because the probiotic as used in the clinical trial does not cause harm. Study collaborators in Thailand are from Rajamangala University of Technology Srivijaya and Prince of Songkla University.P Piewngam et al. Probiotic for pathogen-specific Staphylococcus aureus decolonization a phase 2 randomized placebo-controlled trial. Lancet Microbe DOI 10.1016/S2666-5247(22)00322-6 (2023).P. Piewngam et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. (2018).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/probiotic-markedly-reduces-s-aureus-colonization-phase-2-trial
Researchers develop model for how the brain acquires essential omega-3 fatty acids
Tuesday May 9 2023Findings may aid design of targeted drug delivery into the brain and central nervous system.Omega-3 fatty acids are considered essential because the body cannot make them and must obtain them through foods such as fish nuts and seeds. DHA levels are especially high in the brain and important for a healthy nervous system. Infants obtain DHA from breastmilk or formula and deficiencies of this fatty acid have been linked to problems with learning and memory. To get to the brain omega-3 fatty acids must pass through the blood-brain barrier via the lipid transporter Mfsd2a which is essential for normal brain development. Despite its importance scientists did not know precisely how Mfsd2a transports DHA and other omega-3 fatty acids.In the study the research team provides images of the structure of zebrafish Mfsd2a which is similar to its human counterpart. The snapshots are the first to detail precisely how fatty acids move across the cell membrane. The study team also identified three compartments in Mfsd2a that suggest distinct steps required to move and flip fatty acids through the transporter as opposed to movement through a linear tunnel or along the surface of the protein complex. The findings provide key information on how Mfsd2a transports omega-3 fatty acids into the brain and may enable researchers to optimize drug delivery via this route. The study also provides foundational knowledge on how other members of this transporter family called the major facilitator superfamily (MFS) regulate important cellular functions.Doreen Matthies Ph.D. Head and Louis Tung Faat Lai Ph.D. postdoctoral fellow Unit on Structural Biology are available for interviews.To arrange an interview with Dr. Matthies and/or Dr. Lai please e-mail nichdpress@mail.nih.gov or call 301-496-5133.Nguyen C. Lei H.-T. Lai L.T.F. et. al. Lipid flipping in the omega-3 fatty-acid transporter. Nature Communications DOI 10.1038/s41467-023-37702-7 (2023)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/researchers-develop-model-how-brain-acquires-essential-omega-3-fatty-acids
Screening newborns for deadly immune disease saves lives
Tuesday June 20 2023“This study definitively shows that population-wide newborn screening for SCID has made it possible to save the lives of many more children with the disorder than ever before ” said NIAID Acting Director Hugh Auchincloss M.D. “We hope these findings will encourage more countries to screen newborns for this devastating disease.”SCID is a rare disorder caused by mutations in genes involved in the development and function of infection-fighting immune cells. Infants with SCID appear healthy at birth but are highly susceptible to severe infections. The condition is fatal usually within the first year or two of life unless the infant receives an immune-restoring treatment such as a stem-cell transplant gene therapy or enzyme therapy. Forty to 80 babies in the United States and Canada are diagnosed with SCID annually. The number of babies born with the disorder globally is unknown because most countries do not yet screen for SCID. Incidence ranges from 1 infant per 2 000 live births in regions where inbreeding is common to 1 per 60 000 live births where it is not.PIDTC investigators analyzed data on more than 900 children with confirmed SCID who received treatment for the condition with a transplant of blood-forming stem cells from a non-genetically matched donor at one of 34 sites in the United States or Canada between 1982 and 2018. The researchers examined the five-year overall survival rate of these children from 2010–2018 when state- and province-wide newborn screening was in effect at participating sites compared to earlier time periods. The researchers excluded infants who received stem-cell transplants from genetically matched sibling donors from the analysis because these children had high overall survival rates throughout the study period.The five-year overall survival rate for children with SCID who received a stem-cell transplant from a non-genetically matched donor remained steady at 72% to 73% from 1982 to 2009 despite advances in clinical care then increased to 87% during the years 2010 to 2018. Among children whose disease was first suspected based on the result of newborn screening rather than on illness or family history of SCID and who received a transplant between 2010 and 2018 92.5% survived to age 5 or beyond.Previous research had shown that being younger than 3.5 months at the time of transplant and not having an active infection at that time improved five-year survival rates for children with SCID. An analysis of the PIDTC data demonstrated that both these factors were much more common in the era of newborn screening and drove the increase in the proportion of children who survived to age 5. In addition in 2010–2018 compared to previous decades the percentage of babies with SCID who had never had an infection by the time of transplant was dramatically higher further fueling the survival increase. Moreover regardless of the transplant technique used the percentage of children who survived to age 5 was highest in 2010–2018 compared to earlier decades.Luigi Notarangelo M.D. Christopher Dvorak M.D. Elie Haddad M.D. Ph.D. and Monica Thakar M.D. led the study. Dr. Notarangelo is chief of the NIAID Laboratory of Clinical Immunology and Microbiology. Dr. Dvorak is chief of the Pediatric Allergy Immunology and Bone Marrow Transplantation Division and director of the Pediatric Cellular Therapy Laboratory at University of California San Francisco (UCSF). Dr. Haddad is the associate chair of research and a professor in the Department of Pediatrics at the University of Montreal as well as the head of the Immunology Rheumatology and Allergy Division at CHU Sainte-Justine in Montreal. He also holds the Bank of Montreal chair of pediatric immunology at CHU Sainte-Justine. Dr. Thakar is the medical director of Bone Marrow Transplantation Inpatient Services at Seattle Childrens Hospital as well as an associate professor at both Fred Hutchinson Cancer Center and University of Washington in Seattle.MS Thakar et al. Measuring the effect of newborn screening on survival after haematopoietic cell transplantation for severe combined immunodeficiency a 36-year longitudinal study from the Primary Immune Deficiency Treatment Consortium. The Lancet DOI 10.1016/S0140-6736(23)00731-6 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/screening-newborns-deadly-immune-disease-saves-lives

Researchers find weaker immune response to viral infections in children with mitochondrial disorders
Friday July 7 2023One of the first human studies on how mitochondrial function impacts immune cells to guide future treatments.“Our work is one of the first examples to study how B cells are affected in mitochondrial disease by looking at human patients ” said Eliza Gordon-Lipkin M.D. assistant research physician in NHGRIs Metabolism Infection and Immunity Section and co-first author of the paper.Mitochondria are important components of nearly every cell in the body because they convert food and oxygen into energy. Genomic variants in more than 350 genes have been linked to mitochondrial disorders with varied symptoms depending on which cells are affected.“For children with mitochondrial disorders infections can be life threatening or they can worsen the progression of their disorder ” said Peter McGuire M.B.B.Ch. NHGRI investigator head of the Metabolism Infection and Immunity Section and senior author of the study. “We wanted to understand how immune cells differ in these patients and how that influences their response to infections.”Around 1 in 5 000 people worldwide have a mitochondrial disorder. Examples of mitochondrial disorders are Leighs syndrome which primarily affects the nervous system and Kearns-Sayre syndrome which primarily affects the eyes and heart.While mitochondrial disorders are known to affect organs such as the heart liver and brain less is known how they affect the immune system.Using a genomic technique called single-cell RNA sequencing which analyzes gene activity in different cell types researchers studied immune cells found in blood. These cells include different types of white blood cells that help the body fight infections. During stressful conditions these cells produce a microRNA called mir4485. MicroRNAs are small strings of RNA that help control when and where genes are turned on and off. mir4485 controls cellular pathways that help cells survive.“We think that B cells in these patients undergo cellular stress when they turn into plasma cells and produce antibodies and these B cells then try to survive by producing the microRNA to cope ” said Dr. McGuire. “But the B cells are too fragile due to their limited energy so they are unable to survive the stressful conditions.”Researchers used a technique called VirScan to look at all past viral infections assess how well the immune system fought those infections and see the effects of B cells and plasma cells on antibody production. With a weaker antibody response the immune systems in children with mitochondrial disorders are less able to recognize and neutralize invading viruses and clear infections.Researchers aim to use the results of this study to guide future treatment of patients with mitochondrial disorders noting that more translational studies are needed in this research area.Gordon-Lipkin et al. Primary oxidative phosphorylation defects lead to perturbations in the human B cell repertoire. Frontiers in Immunology. DOI 10.3389/fimmu.2023.1142634. (2023).National Human Genome Research Institute (NHGRI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/researchers-find-weaker-immune-response-viral-infections-children-mitochondrial-disorders

New approach successfully traces genomic variants back to genetic disorders
Thursday January 5 2023“We demonstrated that genotype-first research can work especially for identifying people with rare disorders who otherwise might not have been brought to clinical attention ” says Caralynn Wilczewski Ph.D. a genetic counselor at the National Human Genome Research Institutes (NHGRI) Reverse Phenotyping Core and first author of the paper.Typically to treat genetic conditions researchers first identify patients who are experiencing symptoms then they look for variants in the patients genomes that might explain those findings. However this can lead to bias because the researchers are studying clinical findings based on their understanding of the disorder. The phenotype-first approach limits researchers from understanding the full spectrum of symptoms of the disorders and the associated genomic variants.The study documents three types of discoveries from a genotype-first approach.Second this approach helped researchers find novel symptoms related to a disorder that clinicians previously missed because the patient did not have the typical symptoms. NHGRI researchers identified a person with a genomic variant associated with a known metabolic disorder. Further testing found that the individual had high levels of certain chemicals in their body associated with the disorder despite having only minor symptoms.Third this approach allowed researchers to determine the function of specific genomic variants which has the potential to help clinicians understand newly described disorders. For example in one study NHGRI researchers and their collaborators found that a genomic variant was associated with immune dysfunction at the molecular level in blood cells.“Importantly we provide a framework for other institutions to build research programs that allow for genotype-first studies. With more programs taking this approach we can better study the predictive potential of genomic medicine ” said Clesson Turner M.D. director of NHGRIs Reverse Phenotyping Core and a senior author of the article.The framework includes broad genomic data sharing with the ability to recontact participants explicitly stated during the informed consent process. NHGRI researchers recommend institutions aiming to establish genotype-first centers create strategic plans especially for deciding what genomic findings will be returned which may involve genetic counseling services. Importantly according to the study researchers must actively communicate with study participants to build informed and trusting long-term relationships.“In the future as more researchers adopt this approach we hope to identify more people who may be helped by the availability of their genome sequence especially as more diverse populations join genome-sequencing studies ” says Dr. Wilczewski.National Human Genome Research Institute (NHGRI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/new-approach-successfully-traces-genomic-variants-back-genetic-disorders

Clinical trial of mRNA universal influenza vaccine candidate begins
Monday May 15 2023The trial will enroll up to 50 healthy volunteers aged 18 through 49. Three groups of study participants (10 participants each) will be vaccinated with 10 25 and 50 micrograms of the experimental vaccine respectively. After evaluation of the data to determine an optimum dosage an additional 10 participants will be enrolled to receive the optimum dosage. The study also will include a group of participants who will receive a current quadrivalent seasonal influenza vaccine. This will allow the researchers a point of direct comparison between the immunogenicity and safety of the candidate vaccine and available seasonal flu vaccines. Participants will be regularly evaluated to assess the vaccines safety (and secondarily its efficacy) and will receive follow-up appointments for up to one year after vaccination.Seasonal influenza or flu kills thousands of people in the United States each year. The Centers for Disease Control and Prevention estimates that between 2010 and 2020 between 12 000 and 52 000 people died of flu in the United States annually. Although annual seasonal flu vaccines are valuable tools in controlling the spread and severity of influenza they do not provide immunity against every flu strain. Each year before the flu season begins scientific experts must predict which influenza strains are likely to be most common during the upcoming months and then select three or four of these strains to include in the next seasonal flu vaccine. Vaccine manufacturers then need time to produce and distribute the vaccine—during which the dominant strains of the virus can change in unexpected ways potentially decreasing the efficacy of the vaccine. An effective universal flu vaccine could eliminate these problems by protecting its recipients against a wide variety of strains and ideally providing durable long-term immunity so people would not need to be vaccinated every year.“A universal influenza vaccine would be a major public health achievement and could eliminate the need for both annual development of seasonal influenza vaccines as well as the need for patients to get a flu shot each year ” said Acting NIAID Director Hugh Auchincloss M.D. “Moreover some strains of influenza virus have significant pandemic potential. A universal flu vaccine could serve as an important line of defense against the spread of a future flu pandemic.”The early-stage trial is being conducted through the Collaborative Influenza Vaccine Innovation Centers (CIVICs) program which was created by NIAID in 2019 to support the development of broadly protective and longer-lasting flu vaccines. It is the first investigational universal flu vaccine candidate to be tested by the CIVICs program and was manufactured at the facilities of the Duke Human Vaccine Institute (DHVI) which is a part of the CIVICs program.A similar vaccine developed by researchers at NIAIDs VRC already has shown positive results in early clinical trials. Both vaccines use a specific portion of a flu protein hemagglutinin (HA) to induce a broad immune response against influenza. While one portion of the HA protein known as the head tends to change as the flu virus spreads and evolves a more stable portion known as the stem evolves very slowly and is very similar across many different types of the flu virus. By using the HA stem as the basis for a vaccine researchers hope to induce long-term immunity against a broad range of flu viruses. Unlike the VRCs earlier vaccine the H1ssF-3928 mRNA-LNP vaccine candidate uses a messenger RNA (mRNA) platform. By developing and testing a variety of different platforms for a universal flu vaccine researchers are more likely to find one that is both safe and provides strong and broad immunity against a variety of strains.For more information about the clinical trial visit clinicaltrials.gov using the identifier NCT05755620.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/clinical-trial-mrna-universal-influenza-vaccine-candidate-begins

Low-dose atropine eyedrops no better than placebo for slowing myopia progression
Thursday July 13 2023Use of low-dose atropine eyedrops (concentration 0.01%) was no better than placebo at slowing myopia (nearsightedness) progression and elongation of the eye among children treated for two years according to a randomized controlled trial conducted by the Pediatric Eye Disease Investigator Group (PEDIG) and funded by the National Eye Institute (NEI). The trial aimed to identify an effective way to manage this leading and increasingly common cause of refractive error which can cause serious uncorrectable vision loss later in life. Results from the trial were published in JAMA Ophthalmology.Importantly the findings contradict results from recent trials primarily in East Asia which showed a benefit from 0.01% atropine in slowing myopia.Identifying an optimal approach for preventing high (advanced) myopia is urgently needed given the escalating prevalence of myopia overall and the risk of it progressing to high myopia. By 2030 its predicted that 39 million people in the U.S. will have myopia. By 2050 that number is expected to grow to 44 million in the U.S. and to 50% of the global population.Much stronger concentrations of atropine eyedrops (0.5-1.0%) have long been used by pediatric eye doctors to slow myopia progression. While effective such doses cause light sensitivity and blurry near vision while on the nightly eyedrops. Thus there is interest in clinical studies assessing lower concentrations that have been shown to have fewer side effects.“The absence of a treatment benefit in our U.S.-based study compared with East Asian studies may reflect racial differences in atropine response. The study enrolled fewer Asian children whose myopia progresses more quickly and included Black children whose myopia progresses less quickly compared with other races ” noted the studys lead co-author Michael X. Repka M.D. professor of ophthalmology Johns Hopkins University.For the study 187 children ages 5 to 12 years with low-to-moderate bilateral myopia were randomly assigned to use nightly atropine (0.01%) (125 children) or placebo (62 children) eyedrops for two years. Study participants their parents and the eye care providers were masked to the group assignments. Patient care was provided at 12 study center sites throughout the U.S.After the treatment period and 6 months after treatment stopped there were no significant differences between the groups in terms of changes in degree of myopia compared with baseline. Nor were there significant differences in axial length within the two groups when compared with baseline measurements.“Its possible that a different concentration of atropine is needed for U.S. children to experience a benefit ” noted the studys other lead co-author Katherine K. Weise O.D. professor University of Alabama at Birmingham. “Clinical researchers could evaluate new pharmaceuticals and special wavelengths of light in combination with optical strategies like special glasses or contact lenses to see what works in reducing the progression of myopia.”Among children myopia will stabilize in about half of children around age 16 years and among an increasingly larger percentage as they get older. By their early twenties about 10% of individuals with myopia will continue to grow more nearsighted and by age 24 years that percentage is 4%.“Vision scientists may help us figure out whats different about the myopic eye even among different races and ethnicities to help create new treatment strategies ” she said. It will take a real convergence of eye research to solve the environmental genetic and structural mystery of myopia.”PEDIG is a collaborative network of pediatric ophthalmologists and pediatric optometrists dedicated to conducting multi-center trials on eye disorders that affect children. The trial was funded by the NEI grants EY11751 EY18810 and EY23198. The ClinicalTrials.gov identifier is NCT03334253.For more information about myopia visit https //www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/nearsightedness-myopia.NEI leads the federal governments efforts to eliminate vision loss and improve quality of life through vision research…driving innovation fostering collaboration expanding the vision workforce and educating the public and key stakeholders. NEI supports basic and clinical science programs to develop sight-saving treatments and to broaden opportunities for people with vision impairment. For more information visit https //www.nei.nih.gov.Repka MX Weise KK Chandler DL Wu R Melia BM Manny RE Kehler LAF Jordan CO Raghuram A Summers AI Lee KA Petersen DB Erzurum SA Pang Y Lenhart PD Ticho BH Beck RW Kraker RT Holmes JM Cotter SA on behalf of the Pediatric Eye Disease Investigator Group. “Randomized trial of low-dose atropine eyedrops for myopia control” published July 13 2023 in JAMA Ophthalmology.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/low-dose-atropine-eyedrops-no-better-placebo-slowing-myopia-progression

Long-acting antiretroviral therapy suppresses HIV among people with unstable housing, mental illnesses, substance use disorders
Tuesday February 21 2023“ART has been a medical gamechanger for saving lives as treatment and as a potent prevention tool with Undetectable=Untransmittable or U=U. Yet we have substantial gaps that remain for people who face co-occurring health housing and other socio-economic challenges ” said Carl W. Dieffenbach Ph.D. director of the NIAID Division of AIDS. “Making progress against the HIV pandemic necessitates that societies prioritize reaching those who have historically been left behind yet stand to benefit the most from making newer easier formulations of ART available.”Although there are highly effective options for daily oral ART to treat HIV there are many barriers to adherence including housing or food insecurity untreated mental illnesses substance use disorders transportation challenges legal system involvement and other factors.Long-acting injectable medications which are given every four or eight weeks could help people overcome some of these day-to-day treatment barriers. However the only LA-ART combination regimen approved by the U.S. Food and Drug Administration for people with HIV injectable intramuscular cabotegravir and rilpivirine is approved only for patients who have already achieved viral suppression and are currently on oral ART. As such people who face challenges adhering to daily oral ART also face barriers to accessing LA-ART.To address this gap Dr. Gandhi and her team sought to enroll patients in their study who have historically been underserved including those with high rates of unstable housing mental illnesses and substance use disorders. Participants did not have to be on daily oral ART or have viral suppression to be eligible for the study and to start treatment with the long-acting injectable.Between June 2021 and November 2022 133 study participants with HIV started on LA-ART including 57 people (43%) with untreated or unsuppressed HIV and 76 people (57%) who were virologically suppressed on oral ART. The researchers performed biweekly review of each participants health status and pharmacy staff conducted regular outreach to remind patients of their injection appointments.Among participants who began the study with virologic suppression all (100%) remained suppressed over the period of follow-up. Among participants who did not begin the study with virologic suppression at a median of 33 days 55 out of 57 (96.5%) had achieved virologic suppression. Only two of the 133 study participants did not achieve or maintain viral suppression a rate of 1.5% in line with findings from previous clinical trials that studied LA-ART in people with HIV who had achieved viral suppression on daily oral ART.Participants had a median age of 45 and 88% identified as cisgender men 68% identified as non-white 58% reported having unstable housing 8% reported experiencing homelessness 38% reported having a mental illness and 33% reported substance use.“Our patient population does not look like the patient population that got enrolled in the clinical trials to determine the approval criteria for long-acting ART ” said Dr. Gandhi. “It is the role of researchers to help address disparities through intentionally and proactively including diverse groups in our studies and for this population to have the same successful outcomes as those in the other clinical trials was very important and exciting. We want to have the ability to offer these drugs to patients who stand to benefit the most including those who face challenges adhering to daily treatment.”Together results from three landmark NIAID-funded clinical trials―START SMART and HPTN 052―conclusively demonstrated that starting antiretroviral treatment promptly after HIV diagnosis and continuing it without interruption protects the health of the person living with HIV while preventing transmission of the virus to sexual partners. Yet persistent barriers including stigma often delay the start of ART and reduce adherence among people who face significant health and social challenges.Further clinical trial data on the effectiveness of LA-ART in achieving and sustaining virologic suppression among people who face treatment barriers are needed. An ongoing NIAID-supported clinical trial (The LATITUDE Study) conducted in the AIDS Clinical Trials Group network is using a randomized design to directly compare the efficacy of LA-ART and oral ART regimens among people experiencing adherence challenges.The study authors also note that reaching patients and following up with them requires intensive resources a limitation that should be addressed to make LA-ART more widely available.“The most effective treatments are those that fit into the lives of people who need them. These findings show that with the right support long-acting ART can make it easier for people with HIV who face barriers in adhering to daily oral treatment to keep the virus under control ” said NIMH Director Joshua A. Gordon M.D. Ph.D.For more information on substance and mental health treatment programs in your area call the Substance Abuse and Mental Health Services Administrations National Helpline at 1-800-662-HELP (4357) or visit www.FindTreatment.gov.M Gandhi et al. “High Virologic Suppression Rates on Long-Acting ART in a Safety-Net Clinical Population” (Presentation #518). Abstract from the 2023 Conference on Retroviruses and Opportunistic Infections 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/long-acting-antiretroviral-therapy-suppresses-hiv-among-people-unstable-housing-mental-illnesses-substance-use-disorders

New atlas of human kidney cells to help unlock kidney disease research
Wednesday July 19 2023Due to the complexity of the kidney scientists have struggled to develop kidney models that accurately represent human kidney structures and function. The lack of human kidney models has limited the ability to develop new drugs to treat or prevent kidney disease.The Kidney Tissue Atlas comprises maps of 51 main kidney cell types that include rare and novel cell populations 28 kidney cellular states that represent injury or disease a repository of raw gene data and interactive 3D models of cells and microenvironment relationships created from 45 healthy donor kidneys and 48 kidney disease biopsies. The atlas thus establishes a critical foundation for KPMPs overall goal to help discover new treatments for chronic kidney disease (CKD) and acute kidney injury (AKI) medical conditions that present a significant global health burden. The publicly available data created by KPMP including all 3D renderings and analytical tools can be accessed at atlas.kpmp.org.“KPMPs new atlas represents open public science at its best ” said Dr. Eric Brunskill KPMP program director in NIDDKs Division of Kidney Urologic and Hematologic Diseases. “With the atlas weve created an interactive hypothesis-generating resource for kidney disease investigators and clinicians around the world.”While CKD and AKI have historically been described as single uniform diseases KPMP builds on growing consensus that kidney disease can have several different root causes and disease pathways leading to subgroups of CKD and AKI. Instead of a “one size fits all” approach to treating kidney disease precision medicine explores more personalized treatments. KPMPs kidney atlas is intended to help identify disease subgroups within CKD and AKI leading to the discovery of new and possibly individualized ways to treat CKD and AKI.“KPMP brings together the best of new technology patient engagement and partnership and represents an evolution in the way we think about kidney disease ” said NIDDK Director Dr. Griffin P. Rodgers. “Were confident the Kidney Tissue Atlas will help us discover new ways to get the right kidney disease treatment to the right patient at the right time.”Data related to this research are available for request at the NIDDK Central Repository.Lake BB et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature. 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/new-atlas-human-kidney-cells-help-unlock-kidney-disease-research

Being hospitalized with acute kidney injury may increase risk for rehospitalization and death
Thursday April 27 2023According to the findings people who had been hospitalized with AKI whether or not they had pre-existing kidney disease were 62% more likely to be readmitted to the hospital for any reason and 266% more likely to die of any cause within 90 days after discharge from the hospital. During the year following discharge those hospitalized with AKI were re-hospitalized nearly 60% more often than those hospitalized without AKI and more than twice as likely to die. Heart failure sepsis and pneumonia were among the most common causes for readmission after discharge with an AKI hospitalization.“We hope this study leads to a growing awareness about the dramatic increased adverse risks after a hospitalization with AKI – outcomes that could substantially affect health ” said Dr. Ivonne Schulman program director at NIDDKs Division of Kidney Urologic and Hematologic Diseases and one of the papers co-authors. “There currently is no standard of care for people after being hospitalized with AKI and this information could help get us there.”The researchers analyzed data from nearly 1 million people in a national health insurance claims database comparing around 470 000 patients who had a hospitalization with an AKI diagnosis with the same number of patients hospitalized without an AKI diagnosis. The two groups were matched on other characteristics such as pre-existing medical conditions sex race and ethnicity.AKI is more common in people with medical conditions such as diabetes high blood pressure heart disease or chronic kidney disease. AKI can have different causes including overuse or misuse of certain medications or injury to the kidneys from systemic infections. It can also progress into chronic kidney disease a long-term loss of kidney function possibly leading to the need for kidney transplant or dialysis.“Monitoring people with AKI in the weeks after hospital discharge may be critical in preventing future adverse health outcomes ” said NIDDK Director Dr. Griffin P. Rodgers. “These findings present an opportunity for further research to develop and test interventions designed to reduce the risks associated with AKI.”Schulman IH et al. Readmission and mortality after hospitalization with acute kidney injury. American Journal of Kidney Diseases. 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/being-hospitalized-acute-kidney-injury-may-increase-risk-rehospitalization-death

Xylazine appears to worsen the life-threatening effects of opioids in rats
Tuesday June 20 2023Research has shown xylazine is often added to illicit opioids including fentanyl and that xylazine has been increasingly detected in the illicit opioid supply. While some people knowingly use fentanyl and xylazine in combination many people do not know if the drugs they plan to use contain fentanyl xylazine or both. This combination can be extremely dangerous and in April 2023 the U.S. government declared fentanyl adulterated or associated with xylazine as an emerging drug threat.“Drug mixtures containing both xylazine and opioids such as fentanyl demonstrate how rapidly the drug supply can change and how dangerous products can proliferate despite rampant overdose deaths ” said Nora Volkow M.D. director of NIDA. “Understanding the mechanisms behind how xylazine contributes to drug overdoses is essential to enable us to develop interventions that can reverse overdoses and save lives. In the meantime naloxone an opioid overdose reversal medication should always be administered in the event of an overdose because xylazine is most often combined with opioids such as fentanyl.”Xylazine is known to cause sedation and dangerously slow breathing heart rate and low blood pressure in people. The harms of xylazine and risk of fatal overdose are also known to increase when it is used in combination with other central nervous system depressants like alcohol benzodiazepines and opioids like fentanyl or heroin. However the mechanisms underlying the effects of xylazine and its interaction with opioid drugs are largely unknown. In this study a research team led by the NIDA Intramural Research Program conducted a series of experiments in rats to better understand the effects of xylazine as an adulterant to fentanyl and heroin.For the first stage of the study xylazine was administered on its own and in different doses to assess the effects of xylazine on movement temperature and oxygen levels in the brain. Even with low doses of xylazine researchers observed known effects of the drug including sedation muscle relaxation and decreased body temperature. Researchers also observed a modest decrease in brain oxygen levels which was long-lasting and dose-dependent meaning the decrease in oxygen levels in the brain was stronger when more xylazine was administered.In the second phase of the study either fentanyl or heroin was administered to examine the changes in levels of oxygen in the brain after being exposed to these drugs. Researchers then compared the effects between the combination of either fentanyl and xylazine or heroin and xylazine. In contrast to the modest and prolonged decreases in brain oxygen levels observed with xylazine administering fentanyl or heroin on their own generated an initial rapid and strong decrease in brain oxygen levels resulting from slowed breathing. This was followed by a slower and more prolonged brain oxygen increase.However when the xylazine-fentanyl mixture or the xylazine-heroin mixture were administered the rebounding increase in oxygen to the brain was eliminated and the brain oxygen levels therefore remained lower for a longer period compared to fentanyl or heroin alone. In addition the xylazine-heroin mixture resulted in a much stronger and more prolonged initial decrease in brain oxygen compared to heroin alone.These findings suggest that the addition of xylazine to fentanyl or heroin disrupts the mechanism that the brain uses to counteract a rapid loss of oxygen after being exposed to opioid drugs. The authors therefore hypothesize that xylazine is contributing to overdose deaths involving opioids.“The risks that people face from a drug contaminated with fentanyl are very concerning and this study provides evidence to suggest that the addition of xylazine is exacerbating those risks ” said Eugene A. Kiyatkin M.D. Ph.D. lead author on the paper and senior associate scientist in the NIDA Behavioral Neuroscience Branch. “Further research is needed to explore how these observations may apply in humans and to continue to parse the complex role of illicit drug combinations with xylazine and risk of overdose.”The United States has experienced a massive change in its illicit drug supply over the past several years most notably through the rapid expansion of illicit fentanyl a cheap very potent synthetic opioid drug. There has been a corresponding dramatic increase in overdose deaths which have now plateaued at more than 100 000 people dying in the U.S. annually.For more information on substance and mental health treatment programs in your area call the free and confidential.S Choi MR Irwin & E Kiyatkin. Xylazine effects on opioid-induced brain hypoxia. Psychopharmacology. DOI 10.1007/s00213-023-06390-y (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/xylazine-appears-worsen-life-threatening-effects-opioids-rats

“Eat, Sleep, Console” reduces hospital stay and need for medication among opioid-exposed infants
Monday May 1 2023Opioid-exposed newborns may develop symptoms of neonatal opioid withdrawal syndrome (NOWS) which includes tremors; excessive crying and irritability; and problems with sleeping and feeding. For the past 50 years FNAST has been the traditional assessment tool for infants with NOWS. FNAST is an extensive scoring system that assesses signs of withdrawal in more than 20 areas. Concerns have been raised about its subjectivity and overestimation of the need for opioid medication.The ESC care approach was developed about eight years ago and is growing in popularity in some nurseries but has not been rigorously tested in a large population. ESC assessments are centered on an infants ability to eat sleep and be consoled and the approach keeps mother and baby together enabling families to play a larger role in the care of their infants. However the widespread adoption of ESC without solid evidence of effectiveness and safety has raised concerns about potentially undertreating infants or discharging them prematurely.In the current study researchers enrolled 1 305 infants across 26 U.S. hospitals. The hospitals were randomized to transition from usual care with FNAST to the ESC care approach at different times. On average infants cared for with ESC were medically ready for discharge after an average of 8.2 days whereas infants cared for with FNAST were medically ready for discharge after 14.9 days with an average difference of 6.7 days between the two groups.The study also evaluated whether newborns received opioid medication to manage their symptoms. Infants cared for with ESC were about 63% less likely to receive opioids (19.5% in the ESC group received opioid medication compared to 52% in the FNAST group). Safety outcomes at three months of age were similar between both groups.ACT NOW is a collaborative effort between NICHD and ECHO to improve treatment and care of infants and children exposed to opioids during pregnancy. The program uses NICHDs Neonatal Research Network and ECHOs Institutional Development Award (IDeA) States Pediatric Clinical Trials Network to ensure a geographically and racially diverse group of participants.Young LW. et al. Eat sleep console approach or usual care for neonatal opioid withdrawal. New England Journal of Medicine DOI 10.1056/NEJMoa2214470 (2023)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/eat-sleep-console-reduces-hospital-stay-need-medication-among-opioid-exposed-infants
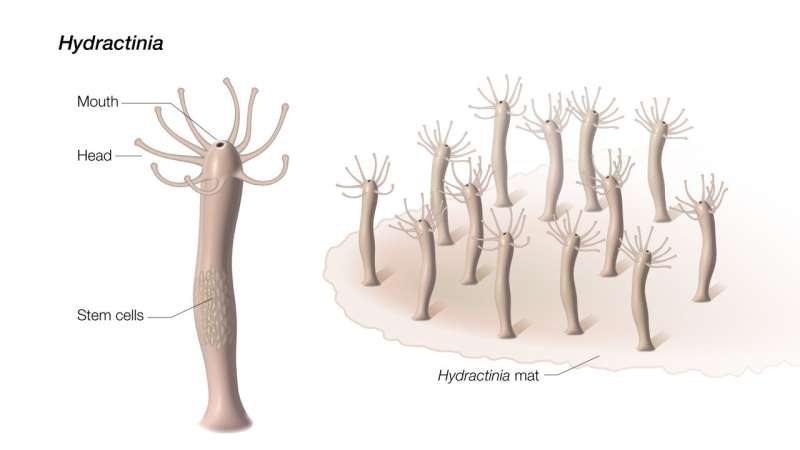
Scientists discover clues to aging and healing from a squishy sea creature
Friday June 30 2023A relative of jellyfish and corals regrows its entire body with help from “aging” cells.Untangling the evolutionary origins of fundamental biological processes such as aging and healing is essential to understanding human health and disease. Humans have some capacity to regenerate like healing a broken bone or even regrowing a damaged liver. Some other animals such as salamanders and zebrafish can replace entire limbs and replenish a variety of organs. However animals with simple bodies like Hydractinia often have the most extreme regenerative abilities such as growing a whole new body from a tissue fragment.A regenerative role for senescence stands in contrast to findings in human cells. “Most studies on senescence are related to chronic inflammation cancer and age-related diseases ” said Andy Baxevanis Ph.D. senior scientist at NHGRI and an author of the study. “Typically in humans senescent cells stay senescent and these cells cause chronic inflammation and induce aging in adjacent cells. From animals like Hydractinia we can learn about how senescence can be beneficial and expand our understanding of aging and healing.”Previously researchers found that Hydractinia has a special group of stem cells for regeneration. Stem cells can transform into other types of cells and are therefore useful for creating new body parts. In humans stem cells mainly act in development but highly regenerative organisms like Hydractinia use stem cells throughout their lifetimes. Hydractinia stores its regeneration-driving stem cells in the lower trunk of its body. However when the researchers remove the mouth — a part far from where the stem cells reside — the mouth grows a new body. Unlike human cells which are locked in their fates the adult cells of some highly regenerative organisms can revert into stem cells when the organism is wounded though this process is not well understood. The researchers therefore theorized that Hydractinia must generate new stem cells and searched for molecular signals that could be directing this process.When RNA sequencing pointed to senescence the researchers scanned the genome of Hydractinia for sequences like those of senescence-related genes in humans. Of the three genes they identified one was “turned on” in cells near the site where the animal was cut. When the researchers deleted this gene the animals ability to develop senescent cells was blocked and without the senescent cells the animals did not develop new stem cells and could not regenerate.The researchers tracked the senescent cells in Hydractinia to find how this animal circumvents the harmful effects of senescence. Unexpectedly the animals ejected the senescent cells out of their mouths. While humans cant get rid of aging cells that easily the roles of senescence-related genes in Hydractinia suggest how the process of aging evolved.We humans last shared an ancestor with Hydractinia — and its close relatives jellyfish and corals — over 600 million years ago and these animals dont age at all. Because of these factors Hydractinia can provide crucial insights about our earliest animal ancestors. Therefore the researchers theorize that regeneration may have been the original function of senescence in the first animals.“We still dont understand how senescent cells trigger regeneration or how widespread this process is in the animal kingdom ” said Dr. Baxevanis. “Fortunately by studying some of our most distant animal relatives we can start to unravel some of the secrets of regeneration and aging — secrets that may ultimately advance the field of regenerative medicine and the study of age-related diseases as well.”Miguel Salinas-Saavedra Febrimarsa Gabriel Krasovec Helen R. Horkan Andreas D. Baxevanis Uri Frank. Senescence-induced cellular reprogramming drives cnidarian whole-body regeneration. Cell Reports 2023 DOI https //doi.org/10.1016/j.celrep.2023.112687.National Human Genome Research Institute (NHGRI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/scientists-discover-clues-aging-healing-squishy-sea-creature

Researchers show how a tumor cell’s location and environment affect its identity
Friday June 23 2023New approach could provide insights into cancer progression and treatment response leading to more precise therapies.The approach pairs the use of a technology to reveal the genetic activity of single cells within a tumor with fluorescent dyes that spread into tumors. The work could allow researchers to study how the same diseases can vary in people and progress differently. This research could help clinicians identify treatment strategies focused on specific areas in tumors which could lead to better therapies for cancers and other diseases. The team reported its results June 21 in Cell Systems.“Its commonly accepted that a cells location and surrounding environment influence the cells identity ” said Craig Thomas Ph.D. a translational scientist at NCATS. “Two cells can be genetically identical but have different cellular identities meaning different genes are turned on because of their location and environment. Our goal was to establish a straightforward method to study this concept in multiple settings.”The new system called Segmentation by Exogenous Perfusion or SEEP takes advantage of a dye that diffuses into cells throughout a tumor at a definable rate. Measuring how much dye gets into individual tumor cells provides information on the cells location and specifically its access to the outside environment. Using computational methods the researchers linked this information to cells gene activity allowing the scientists to connect the cells identities with their location.“Understanding the relation of cells to each other and the effects of their positions in space has been a fundamental question in cancer neurological disorders and other areas ” said co-author Tuomas Knowles Ph.D. at the University of Cambridge.Researchers used three types of 3-D laboratory models — spheroids organoids and mouse models — created from human ovarian cancer cells. Spheroids are 3D clusters of cells grown in a lab dish that can mimic some traits of organs and tissues. Organoids also grown in a dish are more complex 3-D models that more closely mimic organ and tissue function and structure. In the mouse models researchers implanted human ovarian cancer cells to form tumors.“Its critical to understand that not every cell in a tumor will be exposed to a drug in the same way ” Knowles said. “A cancer drug might kill the cells on the surface of a tumor but the cells in the middle are different and affected differently. Thats likely contributing to why some therapies fail.”The SEEP method revealed that tumor cells near the tumor surface were more likely to undergo cell division than cells closer to the tumor center. Cells on the surface of tumors also turn on genes to protect them from immune system responses. Not surprisingly these gene responses are linked to how the tumor hides from the bodys immune defenses.Researchers were surprised at the differences in gene activity between cells on or near the surface and those farther inside the ovarian cancer tumor models. The findings could help scientists better understand how tumors are structured. Such information could lead to improved treatments. One possible cancer treatment method could be to target cells likely to be affected in different areas of tumors.“Certain tumor cell types are susceptible to certain therapies ” noted first author and Harvard University medical student David Morse Ph.D. “Knowing where cells are located and their levels of accessibility in the tumor could help us decide how to use drugs in combination. It could help tell us how long to give a drug and when to move on to other therapies.”About the National Center for Advancing Translational Sciences (NCATS) NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about how NCATS helps shorten the journey from scientific observation to clinical intervention visit https //ncats.nih.gov.DB Morse et al. Positional influence on cellular transcriptional identity revealed through spatially segmented single-cell transcriptomics. Cell Systems DOI https //doi.org/10.1016/j.cels.2023.05.003National Center for Advancing Translational Sciences (NCATS)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/researchers-show-how-tumor-cell-s-location-environment-affect-its-identity

Blood test identifies acute myeloid leukemia patients at greater risk for relapse after bone marrow transplant
Tuesday March 7 2023A small portion of adults in remission from a deadly blood cancer had persisting mutations that were detected which predicted their risk of death from having the cancer return.About 20 000 adults in the United States are diagnosed each year with AML a deadly blood cancer and about one in three live past five years. A bone marrow transplant which replaces unhealthy blood-forming cells with healthy cells from a donor often improves these chances. However research has shown that lingering traces of leukemia can make a transplant less effective.Researchers in the current study wanted to show that screening patients in remission for evidence of low levels of leukemia using standardized genetic testing could better predict their three-year risks for relapse and survival. To do that they used ultra-deep DNA sequencing technology to screen blood samples from 1 075 adults in remission from AML. All were preparing to have a bone marrow transplant. The study samples were provided through donations to the Center for International Blood and Marrow Transplant Research.After screening adults with variants commonly associated with AML researchers showed that the two most common mutations in AML — NPM1 and FLT3-ITD — could be used to track residual leukemia. Among 822 adults with these variants detectable at initial diagnosis 142 adults — about 1 in 6 — were found to still have residual traces of these mutations after therapy despite being classified as in remission.The researchers found the outcomes for these patients striking. Nearly 70% of patients with the lingering NPM1 and FLT3-ITD mutations relapsed and just 39% survived after three years. In comparison only 21% of adults without this evidence of trace leukemia relapsed after three years and 63% survived. “If Im one of six people waiting in a doctors office and were all being told were going in for a transplant and weve got the same risk I want to know if Im actually one of those five who has a 20% chance of relapse or if I am the one with a 70% chance of relapse ” said study lead Christopher S. Hourigan M.D. D.Phil. senior investigator and chief of the Laboratory of Myeloid Malignancies part of the National Heart Lung and Blood Institutes (NHLBI) Intramural Program.“Having this increased risk for relapse may not impact a persons decision about having a bone marrow transplant but it could influence their next steps in care ” Hourigan said. “For that one person out of six the transplant often isnt going to be enough. Other options might include also enrolling in a clinical research trial or considering additional or different therapies.” “This study confirms prior research and provides new important data showing why testing for residual disease before a transplant is critical ” said Rear Admiral Richard Childs M.D. clinical director and acting scientific director of NHLBI. “This information can also empower physicians to tailor transplant strategies including considering different pre-transplant conditioning regimens and chemotherapies to reduce an AML patients risk for relapse and improve their long-term chance for survival.” In their analysis the researchers also observed that adults with persistent mutations but who were younger than age 60 and received higher doses of chemotherapy and/or radiotherapy as part of their transplant preparation were more likely to remain cancer free after three years than those receiving lower doses.They also found that adults who didnt receive stronger treatment before the transplant which is now recommended as part of clinical guidelines did better when this lower-dose therapy included a chemotherapy drug melphalan. However more research is needed to evaluate these potential benefits and of other treatments including targeted therapy for the FLT3-ITD mutation.“Finding bold and innovative approaches including precision therapy for AML is essential to the Biden Administrations goal to cut the death rate from cancer in half within the next 25 years ” said James H. Doroshow M.D. Deputy Director for Clinical and Translational Research at the National Cancer Institute (NCI). AML accounts for 1% of all new cancer cases and adults ages 65 and older are more likely to receive a diagnosis.To learn more about AML visit https //www.cancer.gov/types/leukemia/patient/adult-aml-treatment-pdq and https //www.nhlbi.nih.gov/science/myeloid-malignancies.Dillon LW Gui G Page KM et al. DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA. 2023; doi 10.1001/jama.2023.1363.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/blood-test-identifies-acute-myeloid-leukemia-patients-greater-risk-relapse-after-bone-marrow-transplant

Brain signatures for chronic pain identified in a small group of individuals
Monday May 22 2023For the first time researchers have recorded pain-related data from inside the brain of individuals with chronic pain disorders caused by stroke or amputation (phantom limb pain). A long sought-after goal has been to understand how pain is represented by brain activity and how to modulate that activity to relieve suffering from chronic pain. Data were collected over months while patients were at home and they were analyzed using machine learning tools. Doing so the researchers identified an area of the brain associated with chronic pain and objective biomarkers of chronic pain in individual patients.Chronic pain is one of the largest contributors to disability worldwide. Neuropathic pain is caused by damage to the nervous system itself. It most commonly occurs due to injury to the nerves in our bodies but for the individuals in this study their pain is thought to originate from the brain itself. This kind of pain does not respond well to current treatments and can be debilitating for people living with it.“When you think about it pain is one of the most fundamental experiences an organism can have ” said Prasad Shirvalkar M.D. Ph.D. associate professor of anesthesia and neurological surgery at the University of California San Francisco and lead author of this study. “Despite this there is still so much we dont understand about how pain works. By developing better tools to study and potentially affect pain responses in the brain we hope to provide options to people living with chronic pain conditions.”Traditionally researchers gather data about chronic pain through self-reports from those living with the condition. Examples of this type of data include questionnaires about pain intensity and emotional impact of pain. This study however also looked directly at changes in brain activity in two regions where pain responses are thought to occur—the anterior cingulate cortex (ACC) and the orbitofrontal cortex (OFC)—as participants reported their current levels of chronic pain.“Functional MRI studies show that the ACC and OFC regions of the brain light up during acute pain experiments. We were interested to see whether these regions also played a role in how the brain processes chronic pain ” said Dr. Shirvalkar. “We were most interested in questions like how pain changes over time and what brain signals might correspond to or predict high levels of chronic pain?”Four participants three with post-stroke pain and one with phantom limb pain were surgically implanted with electrodes targeting their ACC and OFC. Several times a day each participant was asked to answer questions related to how they would rate the pain they were experiencing including strength type of pain and how their level of pain was making them feel emotionally. They would then initiate a brain recording by clicking a remote-control device which provided a snapshot of the activity in the ACC and OFC at that exact moment. Using machine learning analyses the research team was able to use activity in the OFC to predict the participants chronic pain state.In a separate study the researchers looked at how the ACC and OFC responded to acute pain which was caused by applying heat to areas of the participants bodies. In two of the four patients brain activity could again predict pain responses but in this case the ACC appeared to be the region most involved. This suggests that the brain processes acute vs. chronic pain differently though more studies are needed given that data from only two participants were used in this comparison.This study represents an initial step towards uncovering the patterns of brain activity that underly our perception of pain. Identifying such a pain signature will enable the development of new therapies that can alter brain activity to relieve suffering due to chronic pain. The most immediate benefit may be in informing ongoing studies in HEAL and BRAIN to employ deep brain stimulation (DBS) to treat chronic pain. Ongoing and future work involving more participants will be key in determining whether different pain conditions share the OFC activity seen in these patients or how the signatures differ among persons with different pain conditions.More modern approaches to DBS that fine-tune the stimulation based on activity biomarkers from the brain have been used to successfully treat some brain disorders including Parkinsons disease and major depressive disorder but those successes have required well-established brain biomarkers. For conditions such as chronic pain the identification of biomarkers is in the early stages.NINDS is the nations leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.Shirvalkar P. Prosky J. Chin G. et al. “Prediction of Chronic Pain State Using Intracranial Neural Biomarkers” Nature Neuroscience May 22 2023. DOI 10.1038/s41593-023-01338-zU.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/brain-signatures-chronic-pain-identified-small-group-individuals

Young men at highest risk of schizophrenia linked with cannabis use disorder
Thursday May 4 2023Researchers found strong evidence of an association between cannabis use disorder and schizophrenia among men and women though the association was much stronger among young men. Using statistical models the study authors estimated that as many as 30% of cases of schizophrenia among men aged 21-30 might have been prevented by averting cannabis use disorder.Cannabis use disorder and schizophrenia are serious but treatable mental disorders that can profoundly impact peoples lives. People with cannabis use disorder are unable to stop using cannabis despite it causing negative consequences in their lives. Schizophrenia is a serious mental illness that affects how a person thinks feels and behaves. People with schizophrenia may seem like they have lost touch with reality and the symptoms of schizophrenia can make it difficult to participate in usual everyday activities. However effective treatments are available for both cannabis use disorder and schizophrenia.“The entanglement of substance use disorders and mental illnesses is a major public health issue requiring urgent action and support for people who need it ” said NIDA Director and study coauthor Nora Volkow M.D. “As access to potent cannabis products continues to expand it is crucial that we also expand prevention screening and treatment for people who may experience mental illnesses associated with cannabis use. The findings from this study are one step in that direction and can help inform decisions that health care providers may make in caring for patients as well as decisions that individuals may make about their own cannabis use.”Previous studies indicate that rates of daily or near daily cannabis use cannabis use disorder and new schizophrenia diagnoses are higher among men than women and that early frequent cannabis use is associated with an increased risk of developing schizophrenia. However few studies have examined differences in the relationship between cannabis use disorder and schizophrenia across different sex and age groups at the population level.To address this research gap investigators analyzed data from nationwide health registers in Denmark which included health records data from more than 6.9 million people who were aged 16-49 at some point between 1972 and 2021. Using these nationally representative longitudinal data the researchers investigated how the associations between cannabis use disorder and schizophrenia varied by different sex and age groups and how these differences changed over time.Although there are many risk factors associated with schizophrenia in this study researchers sought to estimate the proportion of all schizophrenia cases that may be attributed to cannabis use disorder specifically across sex and age groups at the population level. The study team estimated that 15% of cases of schizophrenia among men aged 16-49 may have been avoided in 2021 by preventing cannabis use disorder in contrast to 4% among women aged 16-49. For young men aged 21-30 they estimated that the proportion of preventable cases of schizophrenia related to cannabis use disorder may be as high as 30%. The authors emphasize that cannabis use disorder appears to be a major modifiable risk factor for schizophrenia at the population level particularly among young men.This study also adds to existing evidence suggesting that the proportion of new schizophrenia cases that may be attributed to cannabis use disorder has consistently increased over the past five decades. The authors note that this increase is likely linked to the higher potency of cannabis and increasing prevalence of diagnosed cannabis use disorder over time.“Increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world while also decreasing the publics perception of its harm. This study adds to our growing understanding that cannabis use is not harmless and that risks are not fixed at one point in time ” said Carsten Hjorthøj Ph.D. lead author of the study and associate professor at the Mental Health Services in the Capital Region of Denmark and at the University of Copenhagen.The authors note that further research is needed to examine potential differences in the potency and frequency of cannabis consumption between young men and women and to examine the mechanisms underlying the higher vulnerability of young men to the effects of cannabis on schizophrenia. The association of cannabis potency with cannabis use disorder and psychosis may help inform public health guidelines; policies on cannabis sales and access; and efforts to effectively prevent screen for and treat cannabis use disorder and schizophrenia.For more information on substance and mental health treatment programs in your area call the free and confidential National Helpline 1-800-662-HELP (4357) or visit www.FindTreatment.gov.C Hjorthøj et al. Association between cannabis use disorder and schizophrenia stronger in young males than in females. Psychological Medicine. DOI 10.1017/S0033291723000880 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/young-men-highest-risk-schizophrenia-linked-cannabis-use-disorder

Risk of developing heart failure much higher in rural areas vs. urban
Wednesday January 25 2023“We did not expect to find a difference of this magnitude in heart failure among rural communities compared to urban communities especially among rural-dwelling Black men ” said Véronique L. Roger M.D. M.P.H. the studys corresponding author and a senior investigator with the Epidemiology and Community Health Branch in NHLBIs Division of Intramural Research. “This study makes it clear that we need tools or interventions specifically designed to prevent heart failure in rural populations particularly among Black men living in these areas.”Researchers from NHLBI and Vanderbilt University Medical Center analyzed data from The Southern Community Cohort Study a long-term health study of adults in the southeastern United States. They compared the rates of new onset heart failure among rural and urban residents in 12 states (Alabama Arkansas Florida Georgia Kentucky Louisiana Mississippi North Carolina South Carolina Tennessee Virginia and West Virginia). The population which included 27 115 adults without heart failure at enrollment were followed for about 13 years. Nearly 20% of participants lived in rural areas; the remainder lived in urban areas. Almost 69% were Black adults recruited from community health centers that care for medically underserved populations.At the end of the study period the researchers found that living in rural America was associated with an increased risk of heart failure among both women and Black men even after adjustment for other cardiovascular risk factors and socioeconomic status. Overall the risk of heart failure was about 19% higher in rural residents than their urban counterparts. However Black men living in rural areas had the highest risk of all — a 34% higher risk of heart failure compared to urban-dwelling Black men.The study showed white women living in rural areas had a 22% increased risk of heart failure compared to white women in urban areas and Black women had an 18% higher risk compared to Black women in urban areas. No association was found between rural living and heart failure risk among white men.The exact reasons behind these rural-urban health disparities are unclear and are still being explored. Researchers said a multitude of factors may be at play including structural racism inequities in access to health care and a dearth of grocery stores that provide affordable and healthy foods among others.“Finding an association between living in rural areas and an increased incidence of heart failure is an important advance especially given its implications for helping to address geographic- gender- and race-based disparities ” said David Goff M.D. Ph.D. director of NHLBIs Division of Cardiovascular Sciences. “We look forward to future studies testing interventions to prevent heart failure in rural populations as we continue to fight heart disease the leading cause of death in the U.S.”Heart failure is a chronic and progressive condition that develops when the heart does not pump enough blood for the bodys needs. Common symptoms include shortness of breath during daily activities or trouble breathing when lying down. The condition which has few treatment options affects about 6.2 million American adults.Heart failure can be prevented by following a heart-healthy lifestyle. NHLBIs Roger who is also a practicing cardiologist noted one of the biggest contributors to heart failure is hypertension or high blood pressure which Black men experience at disproportionately high levels. The condition should be intensively managed by checking blood pressure regularly and taking medications as prescribed. Other ways to reduce heart failure risk include avoiding all forms of tobacco eating healthy and exercising.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Rurality and heart failure The Southern Community Cohort Study. JAMA Cardiology. doi 10.1001/jamacardio.2022.5211National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/risk-developing-heart-failure-much-higher-rural-areas-vs-urban

Anti-poverty programs may help reduce disparities in brain development and mental health symptoms in children
Tuesday May 2 2023The study published in Nature Communications highlights the impact that socioeconomic inequities can have on a childs brain development but demonstrates that this gap can be mitigated through state anti-poverty programs such as Earned Income Tax Credit Temporary Assistance for Needy Families and Medicaid.The findings reflect data from the large multisite Adolescent Brain Cognitive Development Study (ABCD Study) led by NIDA. Researchers from Harvard University in Cambridge Mass.; and Washington University St. Louis analyzed ABCD Study data from more than 10 000 youth across 17 states that differ in their cost of living and anti-poverty policies.Emerging evidence has shown that children from families with lower income relative to children from families with higher income exhibit smaller hippocampal volume. The hippocampus plays a critical role in memory and emotional learning.“Multiple studies have found associations between the brain changes shown in this research and meaningful impacts such as low test scores lack of school readiness and risk factors for mood disorders ” said NIDA Director Nora Volkow M.D. “Investigating the policy factors that are associated with brain development and mental health is an important part of better understanding health inequities that impact people throughout their lives starting in critical periods of development.”Replicating findings from smaller studies the research team first validated that lower family income is associated with smaller hippocampal volume and more symptoms of mental health conditions like anxiety depression aggression impulsivity and inattention among the 9-to 11-year-old participants. They expected these disparities among families with a high versus low income to be exacerbated in more expensive states where the high cost of living places additional strain on low-income households.As hypothesized differences in hippocampal volume between children from high- and low-income families were greater in states with a higher cost of living. However the availability and benefit value of monetary assistance programs in higher cost-of-living states reduced this disparity by 34% and similarly in states with Medicaid expansion the disparity was reduced by 43%. Overall more expensive cost-of-living states with anti-poverty programs in more expensive states had narrower gaps in income-associated differences in brain structure. Similar levels were observed in states with the lowest cost of living.Additionally the income-associated disparity in some mental health symptoms like anxiety and depression was 48% lower in expensive states with larger cash benefits than in states with lower cash benefits. These patterns remained significant when controlling for numerous state-level social economic and political characteristics including population density education equity incarceration rates and gender equity.“The association between brain structure and a low-resource environment is not an inevitability ” said study author David Weissman Ph.D. a postdoctoral fellow in the Stress and Development Lab at Harvard University. “Childrens brains are undergoing substantial development and have enhanced plasticity or capacity for further change based on their environment. These data suggest that policies and programs that work to reduce social and health inequities can directly reach children in disadvantaged environments and help support their mental health.”Weissman and his team – which included Katie McLaughlin Ph.D.; Mark Hatzenbuehler Ph.D.; and Mina Cikara Ph.D. at Harvard and Deanna Barch Ph.D. at Washington University St. Louis – also note that this is a correlational study and there are many other factors for consideration to pinpoint exactly why disparities in brain development and mental health exist along different income levels. In future research they hope to explore impacts of experimental cash-assistance interventions as well as other real-world policy changes to see how they relate to differences in mental health and brain structure in children.The Adolescent Brain Cognitive Development Study and ABCD Study are registered service marks and trademarks respectively of the U.S. Department of Health and Human Services.For more information on substance and mental health treatment programs in your area call the free and confidential National Helpline 1-800-662-HELP (4357) or visit www.FindTreatment.gov.About substance use disorders Substance use disorders are chronic treatable conditions from which people can recover. In 2021 over 46 million people in the United States had at least one substance use disorder. Substance use disorders are defined in part by continued use of substances despite negative consequences. They are also relapsing conditions in which periods of abstinence (not using substances) can be followed by a return to use. Stigma can make individuals with substance use disorders less likely to seek treatment. Using preferred language can help accurately report on substance use and addiction. View NIDAs online guideDG Weissman et al. State-level macro-economic factors moderate the association of low income with brain structure and mental health in U.S. children. Nature Communications. DOI 10.1038/s41467-023-37778-1 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/anti-poverty-programs-may-help-reduce-disparities-brain-development-mental-health-symptoms-children

Intraocular corticosteroids best for treating complications of chronic inflammatory eye condition
Tuesday June 13 2023“Prior to this study we didnt know the best treatment for persistent or recurrent macular edema a major cause of vision loss in people with uveitis ” said Douglas A. Jabs M.D. Johns Hopkins Bloomberg School of Public Health Baltimore chair of the study. “This trial strongly indicates that repeat intraocular corticosteroid injections are superior to either intravitreal injections of methotrexate or ranibizumab.”Uveitis is a collection of inflammatory conditions that affect the internal tissues of the eye. Uveitis can affect the front of the eye (anterior uveitis) the middle of the eye (intermediate uveitis) the back of the eye (posterior uveitis) or the front middle and back of the eye (panuveitis). Inflammation in the eye can lead to fluid buildup in the central part of the eyes light-sensing retina known as the macula and decrease vision. This fluid buildup called macular edema is a complication of uveitis that often persists or recurs over time despite uveitis treatment.Initial treatment for uveitis-related macular edema seeks to control inflammation and reduce the fluid under the retina. While some patients achieve this goal with oral corticosteroids most patients with macular edema also need intraocular corticosteroid injections. The dexamethasone intraocular implant is one such treatment. However intraocular corticosteroids can raise pressure inside the eye. High intraocular pressure is a key risk factor for glaucoma which can damage the optic nerve and lead to vision loss. Intraocular corticosteroids can also lead to cataract a clouding of the eyes lens which decreases vision.In this study researchers compared three treatments for uveitis-related macular edema an additional intraocular corticosteroid injection an injection of the anti-vascular endothelial growth factor (anti-VEGF) drug ranibizumab or an injection of the anti-inflammatory drug methotrexate. Anti-VEGF injections are used to treat age-related macular degeneration as well as macular edema due to other causes such as diabetic retinopathy. Earlier small pilot studies suggested that ranibizumab injections and the anti-inflammatory effects of methotrexate might help reduce uveitis-related macular edema.The clinical trial enrolled 194 participants (225 study eyes) with well-controlled uveitis but persistent or recurrent macular edema. Sixty-five participants received a dexamethasone corticosteroid 65 participants received methotrexate and 64 participants received ranibizumab. The study took place at 33 clinical centers located across the United States the United Kingdom Australia and India. All participants had previously received at least one intravitreal corticosteroid injection for uveitis-related macular edema.The injection schedules for each group were based on how each treatment is generally used in clinical practice. The corticosteroid group participants received one dexamethasone implant injection at baseline and if the macular edema had not resolved another injection at eight weeks. The methotrexate group received one injection at baseline then repeat injections at four and eight weeks if macular edema did not resolve. The ranibizumab group received injections at baseline four weeks and eight weeks even if their macular edema resolved.After 12 weeks all three groups showed reductions in retinal swelling. Reduction was greatest in the dexamethasone group compared to the other two (35% reduction for corticosteroid; 20% for ranibizumab; 11% for methotrexate). In addition only the corticosteroid group showed improvement in vision nearly five letters—about one row on an eye chart. The corticosteroid group did have more occurrences of mild increases in intraocular pressure but rises to high levels were infrequent (<10%) in all three groups.“Intraocular corticosteroid treatment remains the most effective therapy for uveitis-related macular edema ” said Nisha Acharya M.D. University of California San Francisco the lead author of the study. “The vision gains in participants who received the corticosteroid treatment were very promising.”This study was funded by NEI. Allergan and Genentech provided a portion of the dexamethasone implants and ranibizumab respectively. Clinical Trial number NCT02623426.NEI leads the federal governments research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information visit https //www.nei.nih.gov.The Multicenter Uveitis Steroid Treatment Trial (MUST) Research Group. “Intravitreal therapy for uveitic macular edema – ranibizumab vs methotrexate vs the dexamethasone implant The MERIT Trial Results.” Ophthalmology June 13 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/intraocular-corticosteroids-best-treating-complications-chronic-inflammatory-eye-condition
Developing mucosal vaccines for respiratory viruses
Wednesday January 11 2023Unlike the respiratory viruses that cause measles mumps and rubella—for which vaccination or recovery from illness provides decades-long protection against future infection—flu RSV SARS-CoV-2 and “common cold” coronaviruses share several characteristics that enable them to cause repeated re-infections. These include very short incubation periods rapid host-to-host transmission and replication in the nasal mucosa rather than throughout the body. This last feature—non-systemic replication—means these viruses do not stimulate the full force of the adaptive immune response which typically takes a week or more to mount.A next generation of improved vaccines for mucosa-replicating viruses will require advances in understanding on several fronts the authors say. For instance more must be learned about interactions between flu viruses coronaviruses and RSV and the components of the immune response that operate largely or exclusively in the upper respiratory system. Over time these interactions have evolved and led to “immune tolerance ” wherein the human host tolerates transient limited infections by viruses that are generally non-lethal to avoid the destructive consequences of an all-out immune system attack.The authors note that mucosal immunization appears to be an optimal route of vaccination for the viruses of interest when feasible. However to develop useful mucosal vaccines significant knowledge gaps must be filled including finding ideal vaccine formulations; determining dosage size frequency and timing; and developing techniques for overcoming immune tolerance.The NIAID authors urge fellow researchers to “think outside the box” to make strides toward vaccines that can elicit durable protection against these viruses of considerable public health impact. They conclude “we are excited and invigorated that many investigators…are rethinking from the ground up all of our past assumptions and approaches to preventing important respiratory viral diseases and working to find bold new paths forward.”DM Morens et al. Rethinking next-generation vaccines for coronaviruses influenza viruses and other respiratory viruses. Cell Host & Microbe DOI 10.1016/j.chom.2022.11.016 (2023).Co-authors Dr. Jeffery K. Taubenberger Laboratory of Infectious Diseases NIAID and Dr. David M. Morens Office of the Director NIAID are available to discuss their paper.To schedule interviews please contact Anne A. Oplinger (301) 402-1663 NIAIDNews@niaid.nih.gov.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/developing-mucosal-vaccines-respiratory-viruses

Buprenorphine initiation in the ER found safe and effective for individuals with opioid use disorder who use fentanyl
Thursday March 30 2023Withdrawal induced by medications to treat opioid use disorder – called precipitated withdrawal – is a debilitating experience marked by rapid onset of symptoms such as aches nausea and vomiting diarrhea and abdominal cramps that can occur within two hours after the first dose of buprenorphine. Although instances of buprenorphine-precipitated withdrawal have only been reported in relatively small case studies and anecdotal evidence some clinicians and patients worry that the risk of experiencing precipitated withdrawal from buprenorphine might be increased among people who use fentanyl. This has led some clinicians to prescribe buprenorphine at lower doses especially for people using extremely potent illicit opioids such as fentanyl. Because initiating low-dose buprenorphine following initial cessation of illicit opioids can be less effective in relieving these symptoms individuals may be more likely to resume use of illicit opioids.“We are in an overdose crisis and we need to deploy every tool we have to help address it ” said Nora D. Volkow M.D. director of NIDA. “The emergency department is a crucial care setting for people with substance use disorders. This study provides further evidence that all emergency department physicians can and should be using buprenorphine to help individuals take the first steps into treatment and toward recovery.”There has been an urgent need to better understand how the prevalence of fentanyl in the drug supply affects the process of addiction treatment for people with opioid use disorder. The study addressed this question prospectively by analyzing data from 1 200 individuals at 28 U.S. emergency departments participating in an ongoing clinical trial. The trial is comparing the relative impact of a weekly extended-release buprenorphine injection at a dose of 24 milligrams versus daily administration of 8 to 16 mg buprenorphine as a tablet or film.The trial recruited adult patients with untreated moderate to severe opioid use disorder opioid-positive and methadone-negative urine tests and a Clinical Opiate Withdrawal Scale score greater than or equal to 4. In this study precipitated withdrawal was defined as when a patient demonstrated marked escalation by five points or more on the Clinical Opiate Withdrawal Scale within two hours of starting buprenorphine. Researchers found that despite high fentanyl use prevalence – about 70% – among 1 200 people with opioid use disorder precipitated withdrawal occurred in nine out of the total 1 200 people or 0.76% and only 1% of those who had used fentanyl. The rate of precipitated withdrawal was similar to that reported in people using heroin or prescription opioids without fentanyl.“Clinicians should encourage patients with opioid use disorder to take buprenorphine if they need it ” said lead author Gail DOnofrio M.D. professor of emergency medicine at Yale University New Haven Connecticut. “We know that less than 23% of people with opioid use disorder are getting treated for it and we only have a few medications for opioid use disorder that have been found to be very effective for opioid withdrawal to date. If we take away one of these were going to have an even bigger gap between need and treatment. We hope that both clinicians and patients understand that buprenorphine is a safe and effective option.”These findings build upon existing evidence that administering buprenorphine in emergency departments helps people begin addiction treatment and that higher-dose buprenorphine (more than the standard upper limit of 16 mg) is safe and well tolerated in people with opioid use disorder experiencing withdrawal symptoms. They also bolster support for expanding access to buprenorphine. Recent legislation removed barriers to access including the elimination of the X-Waiver in December 2022 and policy efforts have been initiated that maintain COVID-19 era-initiated flexibilities related to prescribing buprenorphine via telehealth evaluations.About substance use disorders Substance use disorders are chronic treatable conditions from which people can recover. In 2021 over 46 million people in the United States had at least one substance use disorder. Substance use disorders are defined in part by continued use of substances despite negative consequences. They are also relapsing conditions in which periods of abstinence (not using substances) can be followed by a return to use. Stigma can make individuals with substance use disorders less likely to seek treatment. Using preferred language can help accurately report on substance use and addiction. View NIDAs online guide.G. DOnofrio et al. Incidence of Precipitated Withdrawal during a Multi-site Emergency Department-Initiated Buprenorphine Clinical Trial in the Era of Fentanyl. JAMA Network Open. DOI 10.1001/jamanetworkopen.2023.6108 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/buprenorphine-initiation-er-found-safe-effective-individuals-opioid-use-disorder-who-use-fentanyl
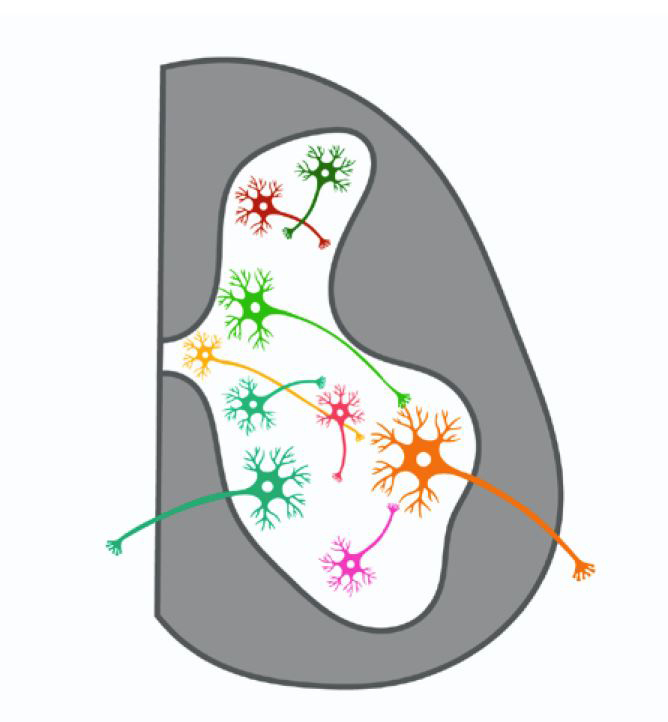
Study identifies features that may make motor neurons vulnerable to ALS
Thursday February 2 2023Human spinal cord cell atlas provides foundation to study neurodegeneration chronic pain and other diseases.The study resulted in a catalog or “atlas” characterizing the diverse community of cell types within the human spinal cord. By examining gene expression at the single-cell level the researchers identified dozens of cell types in the spinal cord and analyzed their molecular profiles. They demonstrated the usefulness of the atlas by looking closely at motor neurons which provide voluntary movement and motor control. Motor neurons which degenerate and die in ALS are large cells with one long extension called an axon―up to a meter long―that conducts signals from the spinal cord to the muscle fiber.The team found that motor neurons are distinguished by a set of genes that may enable the large size of the motor neuron cell body and lengthy axon but also underlie their vulnerability to degeneration. Their molecular profile was defined by genes involved in cytoskeletal structure which gives the cell shape and organizes the structures within; neurofilament genes related to cell size; and genes linked to the onset of ALS. Additional experiments showed that ALS-related genes are also enriched in motor neurons in mice. Taken together the study gives insight into ALS and demonstrates the utility of the spinal cell atlas to study disease and possible interventions.Yadav et al. A Cellular Taxonomy of the Adult Human Spinal Cord. 2023 Feb 1. Neuron. DOI 10.1016/j.neuron.2023.01.007NINDS is the nations leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/study-identifies-features-may-make-motor-neurons-vulnerable-als

FDA-approved drug shows promise in lab models for blinding childhood disease
Wednesday March 29 2023Strategy may speed delivery of therapeutic options relative to time it would take to develop gene therapies.LCA 10 is caused by mutations of the cilia-centrosomal gene (CEP290). Such mutations account for 20% to 25% of all LCA – more than any other gene. In addition to LCA CEP290 mutations can cause multiple syndromic diseases involving a range of organ systems.Using a mouse model of LCA10 and two types of lab-created tissues from stem cells known as organoids the team screened more than 6 000 FDA-approved compounds to identify ones that promoted survival of photoreceptors the types of cells that die in LCA leading to vision loss. The high-throughput screening identified five potential drug candidates including Reserpine an old medication previously used to treat high blood pressure.Observation of the LCA models treated with Reserpine shed light on the underlying biology of retinal ciliopathies suggesting new targets for future exploration. Specifically the models showed a dysregulation of autophagy the process by which cells break down old or abnormal proteins which in this case resulted in abnormal primary cilia a microtubule organelle that protrudes from the surface of most cell types. In LCA10 CEP290 gene mutations cause dysfunction of the primary cilium in retinal cells. Reserpine appeared to partially restore autophagy resulting in improved primary cilium assembly.Reserpine targets dysregulated intracellular signaling pathways downstream of the primary cilium. Such a treatment strategy could potentially address retinal ciliopathies caused by many of the more than 160 disease-causing genes regardless of the specific gene involved. Thats in contrast to gene therapy which requires a very expensive and labor-intensive process to tailor an individual gene-based therapeutic approach for each mutation.This work was supported by the NEI Intramural Research Program (ZIAEY000450 and ZIAEY000546) and the National Center for Advancing Translational Sciences Intramural Research Program (ZIATR000018-06)Anand Swaroop Ph.D. senior investigator and chief of the NEI Neurobiology Neurodegeneration and Repair LaboratoryTo schedule interviews with Dr. Swaroop contact NEI at neinews@nei.nih.govChen HY Swaroop M Papal S Mondal AK Song HB Campello L Tawa GJ Regent F Shimada H Nagashima K de Val N Jacobson SG Zheng W Swaroop A. “Reserpine maintains photoreceptor survival in retinal ciliopathy by resolving proteostasis imbalance and ciliogenesis defects ” eLife March 28 2023 https //doi.org/10.7554/eLife.83205About the NEI NEI leads the federal governments efforts to eliminate vision loss and improve quality of life through vision research…driving innovation fostering collaboration expanding the vision workforce and educating the public and key stakeholders. NEI supports basic and clinical science programs to develop sight-saving treatments and to broaden opportunities for people with vision impairment. For more information visit https //www.nei.nih.gov.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/fda-approved-drug-shows-promise-lab-models-blinding-childhood-disease

Extremely rare gene variants point to a potential cause of age-related macular degeneration
Monday April 3 2023A study from the National Eye Institute (NEI) identified rare genetic variants that could point to one of the general mechanisms driving age-related macular degeneration (AMD) a common cause of vision loss in older adults. The variants generate malformed proteins that alter the stability of the membrane attack complex (MAC) which may drive a chronic inflammatory response in the retina. NEIThere are many known genetic variants that raise or lower an individuals risk of getting AMD; however the contribution of each of these genetic changes to AMD is small.To discover genetic variants – and proteins – with a direct tie to the disease Anand Swaroop Ph.D. chief of NEIs Neurobiology Neurodegeneration and Repair Laboratory and lead author of the study undertook a collaboration with Michael Klein M.D. a leading AMD clinician at the Oregon Health Sciences University (OHSU) Portland. Klein has collected clinical information for hundreds of patients as well as families with a high number of individuals with AMD. Swaroop Klein and colleagues looked for families carrying very rare AMD-causing variants where the effect of the gene variant is very strong and where the variant directly affects protein structure and function. This type of rare variant can reveal the root cause of disease.“While we have known about many genetic variants that affect AMD risk only a few have pointed directly to protein alterations that can cause AMD ” said Swaroop. “By looking at large families with ultra-rare variants that track closely with disease across generations we found two proteins that may directly be the driving force behind AMD pathology in affected patients. These proteins could be targets for future drugs.” While there are currently some treatments to slow vision loss for people with the wet form of AMD there is no treatment for most patients and no cure for the disease.Swaroop Klein and colleagues found that in four families individuals with AMD have mutations in one of two proteins that form one end of MAC C8-alpha and C8-beta. The team found that the variants from the four AMD families all affected the ability of the C8 proteins to stick to each other which may alter how MAC behaves in the eyes retina.MAC forms a circular pore closed at one end by the C8 proteins; the MAC pore permits the flow of ions through the outer membrane of cells. This pore is the final step in the complement cascade a part of the immune system that helps the body defend against pathogens. Although scientists initially thought that MACs only function was to insert into bacterial cell membranes and kill the pathogen more recent evidence shows that MAC plays a complex role in regulating inflammatory processes in tissues like the retina.Genetic data from NEIs Age Related Eye Disease Studies have suggested roles for C8 proteins as well as other proteins higher up in the complement cascade in AMD. Because MAC is the final step in the complement cascade variants affecting any of the complement proteins may funnel down to alter MAC function. The researchers believe that either too much or too little stable MAC in the retina may lead to destructive inflammation which in turn drives AMD progression.“Given that MAC is the end of the immune systems complement pathway and because theres such a strong link between these rare variants and disease we think that targeting it may be a more effective strategy to control AMD ” Swaroop said. “With a small molecule drug we might be able to control how strongly MAC drives inflammation and from there slow down progression of AMD.”The research was funded by the National Eye Institute as well as Research to Prevent Blindness the Retina Research Foundation and the Casey Eye Institute Macular Degeneration Center.NEI leads the federal governments research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information visit https //www.nei.nih.gov.Zelinger L Martin TM Advani J Campello L English MA Kwong A Weber C Maykoski J Sergeev YV Fariss R Chew EY Klein ML and Swaroop A. “Ultra-rare complement factor 8 coding variants in families with age-related macular degeneration.” iScience. April 1 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/extremely-rare-gene-variants-point-potential-cause-age-related-macular-degeneration

Disparities identified among patients receiving advanced pulmonary support
Thursday April 6 2023The study published in the Annals of the American Thoracic Society. In ECMO a machine pumps blood out of the body sends it through devices that feed it oxygen and then returns it to the body. It is typically a treatment of last resort and provided to patients who first receive mechanical ventilation a standard type of breathing assistance used in critical care.Researchers reviewed health insurance data from more than 2 million adults with severe respiratory illness between 2016 and 2019 using the Nationwide Readmissions Database. All patients first received mechanical ventilation which in this case was defined as having a breathing tube inserted into the airways to help their bodies receive enough oxygen. Supplemental oxygen can also be provided through a face mask or breathing tubes inserted into the nostrils but this review only looked at advanced breathing assistance. Among adults who received mechanical ventilation 18 725 also received ECMO. After conducting multiple analyses the researchers found that men received ECMO more often than women even if they had the same type of insurance and income level. Men accounted for 64% of patients receiving ECMO compared to 36% of women. In addition men made up 55% of those who just received mechanical ventilation compared to 45% of women.When looking at ECMO based on insurance types 38% of patients had private insurance and 37% had Medicare. However 18% had Medicaid and 7% had other insurance which could include being uninsured. Among patients who only received mechanical ventilation 58% used Medicare 17% used Medicaid 17% used private insurance and 8% had other insurance.Patients from higher income areas accounted for 25% of those who received ECMO as did 25% of patients from lower-income areas. Still just 17% of patients who only received mechanical ventilation came from high-income areas compared to 33% of patients from lower-income areas.“The goal is to really get people thinking about where some disparities within critical care might live ” said Anuj B. Mehta M.D. the first study author and an assistant professor of medicine within the Division of Pulmonary Sciences and Critical Care Medicine at Denver Health and Hospital Authority and the University of Colorado School of Medicine. “The next step is to think about how we can investigate those disparities with better data and better sources which supports the long-term goal of ensuring equitable care.”Mehta a pulmonologist and critical care medicine doctor stressed these findings are associations and do not necessarily mean that doctors intentionally refer some patients over others for advanced care. He and the authors note multiple factors could explain these variations. Implicit bias among health care providers could be one. Patient preferences could be another. Neither one of these factors could be controlled for in this retrospective review the researchers said. Living near or being more likely to be referred to an advanced medical center that provides ECMO could be a third. Since ECMO is not available at all medical centers and can be limited where it is offered about half of all eligible patients receive it. However even after controlling for access to ECMO such as looking at patients who received care at the same hospital the researchers still found disparities. In addition to controlling for gender health insurance and income the researchers assessed other factors to allow for similar comparisons among patients. This included age severity of illness reasons for seeking care regions where they sought care and other health conditions.“These findings add to existing research that shows more work is needed to both understand and alleviate disparities in advanced pulmonary care ” said James P. Kiley Ph.D. the director of the Division of Lung Diseases at the National Heart Lung and Blood Institute (NHLBI). ECMO was created about 50 years ago. Despite its mixed outcomes in prolonging life compared to other types of respiratory care its use continues to expand.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit https //www.nhlbi.nih.gov.Mehta AB Taylor JK Day G et al. Disparities in adult patient selection for extracorporeal membrane oxygenation in the United States A population-level study. Ann Am Thorac Soc. 2023; doi 10.1513/AnnalsATS.202212-1029OC.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/disparities-identified-among-patients-receiving-advanced-pulmonary-support

HIV can persist for years in myeloid cells of people on antiretroviral therapy
Monday March 27 2023“Our findings challenge the prevailing narrative that monocytes are too short-lived to be important in cure efforts ” said study author Rebecca Veenhuis Ph.D. an assistant professor of molecular and comparative pathobiology and of neurology at Johns Hopkins University School of Medicine Baltimore. “Yes the cells are short-lived but our follow-up data show that HIV can persist in monocytes over several years in people who are virally suppressed. The fact that we can detect HIV in these cells over such a long period suggests something is keeping the myeloid reservoir going.”Antiretroviral medications are effective in treating HIV because they prevent the virus from infecting new cells and multiplying. However HIV may still exist in cells that are in a resting or latent state creating an HIV reservoir. CD4 T cells a type of white blood cell are the most well-studied HIV reservoir. Identifying HIV reservoirs is critical to cure efforts as latent HIV can be reactivated if people stop taking antiretroviral medications.Monocytes are immune cells that circulate in the blood for about 3 days before traveling to tissue in various parts of the body including the brain where they can mature into macrophages. To date it has not been clear whether latent HIV in these cells can become active again and infect other cells.“Whats really important in the long run is understanding how monocytes contribute to the tissue macrophage reservoir ” explained Janice Clements Ph.D. senior author on the study and professor of molecular and comparative pathobiology at Johns Hopkins University School of Medicine. “If monocytes can carry virus to the brain or lung or another part of the body and infect resident macrophages that are self-renewing and live almost indefinitely thats a real problem.”In the study Veenhuis Clements and colleagues first measured HIV DNA in myeloid cells in a sample of 30 participants with HIV all of whom were virally suppressed and had been on antiretroviral therapy for at least 5 years. They found detectable levels of HIV genetic material in monocytes and macrophages though the levels were much lower than those observed in CD4 T cells. In some participants the HIV genetic material found in monocytes was intact which suggests it may be capable of infecting other cells if reactivated.They then used the new quantitative method they developed to directly measure viral spread from HIV found in myeloid cells. The researchers isolated monocytes from blood samples taken from 10 participants and nurtured the monocytes in cultures that contained antiretroviral drugs to replicate the participants baseline physical state. After the monocytes differentiated into macrophages the researchers introduced an immune activating agent and then added fresh white bloods cells to allow for the virus to spread to new cells.The researchers collected samples from the cell cultures several times over the next 12 days. They included checkpoints throughout the process to ensure that infected CD4 T cells did not interfere with their measurements.The results showed that cultures from five of the 10 participants had detectable HIV genetic material in monocyte-derived macrophages that could be reactivated to infect other cells and produce more virus. The participants who had these reactivatable reservoirs of HIV in monocyte-derived macrophages had higher overall levels of HIV DNA material.Follow-up data from three participants showed that this reservoir can be long-lived harboring latent HIV for months to several years. These reservoirs were stable and could be reactivated over time indicating that monocyte-derived macrophages could contribute to viral rebound if antiretroviral treatment is disrupted.The researchers note that this study is small and larger studies with more diverse participant pools will be essential to accurately estimate the proportion of people who have latent HIV in myeloid cells. Investigating the mechanisms that replenish the monocyte reservoir over time is a critical next step in this research.Grants MH127981 MH113512-03S1 MH075673 AI131306 AI127142 AI094189 DA050529Veenhuis R. T. Abreu C. M. Costa P. A. G. Ferreira E. A. Ratliff J. Pohlenz L. Shirk E. N. Rubin L. H. Blankson J. N. Gama L. & Clements J. E. (2023). Monocyte-derived macrophages contain long-lived latent HIV reservoirs. Nature Microbiology. https //doi.org/10.1038/s41564-023-01349-3U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/hiv-can-persist-years-myeloid-cells-people-antiretroviral-therapy
Comparison of diuretics shows no difference in heart failure survival
Tuesday January 17 2023Both drugs are diuretics or water pills which help relieve congestion and breathing difficulties caused by fluid buildup in patients with heart failure. Furosemide which was discovered decades ago is the most-used diuretic to treat heart failure. The other drug torsemide is a comparatively newer medicine. Past studies have suggested torsemide might have an advantage over furosemide in reducing deaths due to heart failure but this question remained unresolved.More than 6 million American adults live with heart failure according to the Centers for Disease Control and Prevention. The chronic debilitating condition develops when the heart cant pump enough blood to meet the bodys needs. It is a growing public health problem with upwards of 8 million Americans anticipated to have heart failure in 2030 according to the American Heart Association. The condition is most common in people 65 years or older.For the trial researchers studied 2 859 patients who had been hospitalized with heart failure at 60 medical centers across the United States. They randomly assigned them to a strategy of either furosemide or torsemide and followed them for an average of 17 months to track survival outcomes. The median age of the patients was 65 years. During the follow-up period death occurred in 26.1% of those on torsemide and 26.2% of the patients on furosemide. “Overall our study showed that torsemide did not improve survival compared to furosemide in this high-risk population of patients with heart failure and we also observed similar rates of hospitalization with the two medications ” said study co-leader Robert J. Mentz M.D. chief of the heart failure section in the Division of Cardiology and associate professor of medicine at Duke University Medical Center Durham North Carolina.“Were not saying that patients dont need diuretics. Were saying that theres no difference in the survival benefit of these two therapies ” Mentz noted. “This suggests we should be spending more time focusing on the right diuretic dose for our patients and working to treat patients with therapies that improve clinical outcomes in heart failure.” Mentz pointed out that the death rate for the patients enrolled in the study was high. About a quarter (26%) of individuals in both drug-treatment groups died during the 17-month follow-up period of the study.David Goff M.D. Ph.D. director of the NHLBIs Division of Cardiovascular Sciences agreed that the high death rate among those patients with heart failure during the trial is concerning given the use of good guideline-based treatments during this trial.The trial participants were diverse and included a high proportion of women (36.9%) and Black Americans (33.9%) who are often underrepresented in clinical studies of heart failure.“This study represents an important step in understanding how heart failure treatments affect all groups and may help reduce health disparities associated with this condition ” said Patrice Desvigne-Nickens M.D. a study co-author and a medical officer in the Heart Failure and Arrhythmias Branch in NHLBIs Division of Cardiovascular Sciences.In the past several decades studies have shown that a few medications improve outcomes for patients with heart failure yet further work is needed to consistently use these therapies in eligible patients. There are also important data highlighting opportunities to prevent heart failure through adapting a heart-healthy lifestyle. This includes aiming for a healthy weight getting regular physical activity quitting smoking getting sufficient sleep and managing stress. Other steps include controlling conditions that increase your risk of heart failure such as diabetes and high blood pressure. If you have heart failure see your healthcare provider to help manage your condition.Research reported in this study was funded by cooperative agreements from the NHLBI (U01-HL125478 and U01-HL125511) and ancillary study grants from the NHLBI (R01HL148354-04 and R01HL154768-02). The ClinicalTrials.gov number is NCT03296813.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Comparative effectiveness of torsemide versus furosemide after discharge from heart failure hospitalization. Journal of the American Medical Association (JAMA). DOI 10.1001/jama.2022.23924National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/comparison-diuretics-shows-no-difference-heart-failure-survival
AI tool may speed screening of epilepsy drugs in mice
Friday February 24 2023Machine learning approach automates behavior analysis outperforms human assessment in animal models of epilepsy.Scientists found that this machine learning-assisted 3D video analysis outperformed the traditional approach in which analyses rely on human observation to label the behavioral signs of epilepsy in animal models during seizures. The labor-intensive process requires constant video monitoring of the mice over many days or weeks while recording their brain wave activity with electroencephalography (EEG). The team led by Stanford researchers studied mice with acquired and genetic epilepsies. They found that machine analysis was better able to distinguish epileptic vs non-epileptic mice than trained human observers. The AI program also identified distinct behavioral phenotypes at different points in the development of epilepsy.Notably researchers were able to use the AI program to distinguish different patterns of behavior in mice after they were given one of three anti-epileptic drugs. This demonstrates that the tool could be used for rapid automated anti-epileptic drug testing. Ultimately the use of automated phenotyping for animal studies of the epilepsies could revolutionize how research is done speeding discovery and reducing costs.The machine-learning technology used in the study called MoSeq for Motion Sequencing locates tracks and quantifies the behavior of freely moving mice in each frame of the video. The information is used to train the unsupervised machine learning model to identify repeated motifs of behavior (called “syllables” – e.g. a right turn or headbob to the left). MoSeq predicts the order (or “grammar”) in which syllables occur allowing fast and objective characterization of mouse behavior.Vicky Whittemore Ph.D. program director NINDS Division of Neuroscience. To arrange an interview please contact NINDSPressTeam@ninds.nih.gov.Gschwind et al. Hidden Behavioral Fingerprints in Epilepsy. Neuron. Feb 2023. DOI https //doi.org/10.1016/j.neuron.2023.02.003NINDS is the nations leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/ai-tool-may-speed-screening-epilepsy-drugs-mice

Risk of rehospitalization in younger women after heart attack nearly double that of men
Tuesday May 2 2023“We have shown for the first time that rehospitalizations following heart attacks in women aged 55 and younger are accompanied by certain non-cardiac factors such as depression and low-income that appear more common in women than men and are associated with more adverse outcomes ” said corresponding author Harlan M. Krumholz M.D. a cardiologist and professor of medicine at the Yale School of Medicine New Haven Connecticut. He is also the director of the schools Center for Outcomes Research and Evaluation (CORE). “The study reveals a need for paying greater attention to these non-cardiac risk factors in younger women in order help design better clinical interventions and improve outcomes after discharge for a heart attack.”“Further study of these risk factors could allow doctors and their patients to focus on ways to help improve a womans health after hospital discharge ” said Yuan Lu Sc.D. principal investigator of the study an investigator at CORE and an assistant professor at Yale School of Medicine.Researchers have known for some time that women aged 55 years and younger have about twice the risk of in-hospital death from a heart attack than similarly aged men. However it was unclear whether women also experience a higher risk of cardiovascular and non-cardiovascular complications a year after leaving the hospital following treatment for a heart attack.To know more researchers analyzed data from the NHLBIs VIRGO (Variation in Recovery Role of Gender on Outcomes of Young AMI Patients) study which looks at a broad range of risk factors related to outcomes among women and men who have had heart attacks. The study included 2 979 patients – 2 007 women and 972 men – at 103 U.S. hospitals. The participants were an average age of 48 years and from ethnically and racially diverse populations.The analysis showed that nearly 30% of these patients were rehospitalized in the year after first leaving the hospital following a heart attack. Most of those re-visits peaked within the first month of a patients discharge then slowly declined in subsequent months. The researchers found that women had nearly twice the risk (1.65 times higher risk) of rehospitalization than men.For men and women coronary-related complications — those such as heart attacks and angina that are related to blood vessel blockage – were the leading cause of rehospitalization. Yet the rate of coronary-related complications for women was nearly 1.5 times higher than that of men – driven in large part by risk factors such as obesity and diabetes.The biggest sex disparities showed up in non-cardiac rehospitalizations which were more than twice as high (or 2.10 times higher) in women than men. These were hospitalizations caused by events not related to heart disease or stroke such as digestive problems depression bleeding and pneumonia.The reasons behind these higher non-cardiac rates are unclear but the researchers found a higher percentage of women than men tended to identify as low income (48% vs 31%) and had a higher history of depression (49% vs 24%). While low income is not a medical measure it is often associated with poor health status due to limited access to healthcare. The risk for depression is known to increase following a heart attack and may be a risk factor in higher hospitalization rates due in part to undertreatment of the condition in women. However further studies will be needed to further explore how these factors affect disparate hospitalizations following a heart attack.“Future research on non-cardiac risk factors after hospital discharge following heart attack could lead to the development of targeted strategies that can narrow this equity gap ” said Gina S. Wei M.D. MPH associate director of NHLBIs Division of Cardiovascular Sciences and NHLBIs senior scientific advisor on womens health. “We look forward to more studies in this area.”This study was supported by grant R01HL081153 from the National Heart Lung and Blood Institute and used data from the VIRGO study (NCT00597922).About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Sex Difference in Outcomes of Acute Myocardial Infarction in Young Patients. Journal of the American College of Cardiology. doi 10.1016/j.jacc.2023.03.383National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/risk-rehospitalization-younger-women-after-heart-attack-nearly-double-men

Single-dose antibiotic prevents maternal sepsis and death
Thursday February 9 2023Results from the study which enrolled more than 29 000 women in seven low- and middle-income countries were published today in the New England Journal of Medicine and presented at the Society for Maternal-Fetal Medicines 43rd Annual Pregnancy Meeting San Francisco.Sepsis — a life-threatening complication of bacterial and other infections — is a leading cause of maternal and newborn deaths worldwide especially in low- and middle-income countries. Azithromycin an inexpensive antibiotic effective against a broad range of bacteria is known to reduce maternal infection when given intravenously during cesarean delivery. Two small studies have suggested that giving the oral version of the drug to women who plan to deliver vaginally may reduce maternal or neonatal infection and death.Launched in 2020 A-PLUS enrolled women at NICHD Global Network sites in Bangladesh the Democratic Republic of the Congo Guatemala India Kenya Pakistan and Zambia. Between September 2020 and August 2022 29 278 participants planning to deliver vaginally were randomly assigned to receive either a two-gram dose of oral azithromycin or a placebo during labor.Within the first six weeks after delivery 227 of 14 526 participants (1.6%) who received azithromycin developed sepsis or died compared to 344 of 14 637 (2.4%) in the placebo group. Deaths were rare in both groups. Sepsis occurred in 219 participants in the azithromycin group (1.5%) and 339 in the placebo group (2.3%). Additionally women who received azithromycin were less likely to develop endometritis (infection of the lining of the womb) and other infections. They also had fewer hospital readmissions and unscheduled healthcare visits compared to the placebo group.“Leveraging the infrastructure and expertise of the NICHD Global Network across three continents allowed us to rapidly gather these important data. We hope that our findings will be applied to help improve maternal care in low- and middle-income countries around the globe ” said Alan T.N. Tita M.D. Ph.D. associate dean of Global and Womens Health of the University of Alabama at Birmingham (UAB) Marnix E. Heersink School of Medicine.The trial was co-led by Dr. Tita and Waldemar A. Carlo M.D. also of UAB. Eight U.S. academic institutions and RTI International Research Triangle Park North Carolina which serves as the data coordinating center for the NICHD Global Network collaborated with eight international partners on A-PLUS.Stillbirth newborn sepsis or death within the first four weeks of life occurred with comparable frequencies in the azithromycin and placebo groups. Overall these adverse events occurred for 10.5% of births in the azithromycin group and 10.3% in the placebo group.A-PLUS was originally designed to enroll up to 34 000 women. However based on a recommendation from the studys independent data and safety monitoring committee following a planned interim data analysis the study was stopped early due to the clear maternal benefit of azithromycin.Tita ATN Carlo WA et al. Azithromycin to prevent sepsis or death in women planning a vaginal birth. New England Journal of Medicine DOI 10.1056/NEJMoa2212111 (2023)Tita AT et al. Intrapartum oral azithromycin to prevent maternal and newborn sepsis or death a multinational RCT. 43rd Annual Pregnancy Meeting. Presented Feb. 9 2023U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/single-dose-antibiotic-prevents-maternal-sepsis-death

First-in-human trial of oral drug to remove radioactive contamination begins
Monday May 15 2023Internal radioactive contamination occurs when radioactive elements are absorbed through wounded skin inhaled or ingested. This could happen as the result of a nuclear power plant accident or the detonation of a “dirty bomb” or nuclear weapon. As the atoms of radioactive elements decay they emit ionizing radiation which can damage DNA tissues and organs. One method for reducing the risk of this damage is to remove the radioactive elements from the body as soon as possible after contamination occurs.The Food and Drug Administration has approved two products for removing internal radioactive contamination. These drugs both based on diethylenetriamine pentaacetate (DTPA) are administered intravenously by a healthcare provider and can remove three radioactive elements plutonium americium and curium.In contrast HOPO 14-1 has been formulated as an oral capsule which would be easier than an intravenous drug to stockpile and to deploy and administer during an emergency. Preclinical research has shown that HOPO 14-1 can effectively remove many radioactive contaminants including uranium and neptunium in addition to plutonium americium and curium. These studies also have found that HOPO 14-1 is up to 100 times more effective than DTPA at binding and removing these radioactive elements.NIAID has funded the discovery and development of HOPO 14-1 since 2006. The active pharmaceutical ingredient in the drug is called 3 4 3-LI(1 2-HOPO).The clinical trial is taking place at a site in Plymouth Michigan under the leadership of Sascha N. Goonewardena M.D. a physician investigator at SRIs Clinical Trials Unit and an assistant professor of medicine at the University of Michigan Medical School in Ann Arbor. The study team will enroll 42 healthy participants ages 18 to 65 years in seven groups of six. Each participant in the first group will receive a 100-milligram (mg) dose of HOPO 14-1. The subsequent groups will receive increasingly higher doses of the study drug up to 7500 mg in the final group if lower doses are deemed safe. Participants will undergo intensive safety monitoring and will be followed for 14 days to measure the absorption distribution and elimination of the study drug. Results are expected in 2024.Additional information about the trial is available in ClinicalTrials.gov under study identifier NCT05628961.Andrea DiCarlo-Cohen Ph.D. director of the Radiation and Nuclear Countermeasures Program in the NIAID Division of Allergy Immunology and Transplantation is available to respond to media inquiries about the trial.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/first-human-trial-oral-drug-remove-radioactive-contamination-begins

Obstructive sleep apnea associated with increased risks for long COVID
Thursday May 11 2023Study suggests adults with both the sleep disorder and COVID may benefit from clinical monitoring.The research which came from EHR data of more than 2.2 million Americans with COVID-19 suggests close monitoring after a COVID-19 infection may help adults with sleep apnea. The findings may also strengthen understanding of why some people are more likely to develop the post-viral syndrome after acute infection.“We still have a lot to learn about the long-term effects of this virus but this study could inform clinical care by identifying patients who may benefit from closer monitoring ” said Marishka K. Brown Ph.D. director of the National Center on Sleep Disorders Research at the National Heart Lung and Blood Institute (NHLBI).“People with obstructive sleep apnea should also keep up with their vaccinations to minimize the risk of infection ” said Lorna E. Thorpe Ph.D. M.P.H. the studys senior author and director of the Division of Epidemiology in the Department of Population Health at New York Universitys Grossman School of Medicine New York City.The data for this analysis came from three RECOVER EHR research networks the National COVID Cohort Collaborative (N3C) which included 1.7 million adults; PCORnet® which included 330 000 adults; and PEDSnet a pediatric-focused research network participating in PCORnet which included 102 000 children. All participants included in this analysis had tested positive for COVID-19 between March 2020 and February 2022.Within each network researchers used diagnostic codes from EHRs to identify participants who had obstructive sleep apnea which occurred among 5% of adults and less than 2% of children. They also used machine learning to assess follow-up symptoms and medical visits to determine which people likely had long COVID. About 5% of adults in the N3C study 17% of adults in PCORnet and less than 5% of children in PEDSnet were suspected to have developed long COVID.After controlling for similarities among patients including COVID-19 severity age sex race and ethnicity and underlying medical conditions researchers found adults with obstructive sleep apnea in N3C the largest study were 75% more likely to experience long COVID. For adults in PCORnet the increased odds of having long COVID was 12%. No significant links between sleep apnea and long COVID in children were found after researchers controlled for other medical conditions including obesity.A follow-up analysis with additional patients confirmed these associations – showing a link between obstructive sleep apnea and increased odds for long COVID in adults. “Part of the challenge is that many of the risk factors for sleep apnea are also risk factors for COVID-19 outcomes ” said Thorpe. “We dont know entirely why we are seeing this association.”The researchers also found women in the N3C study had an 89% increased likelihood of having long COVID if they had obstructive sleep apnea compared to a 59% increased chance for men. The underlying associations arent clear. However women diagnosed with obstructive sleep apnea included in this study may have had more severe conditions than men. Severity of obstructive sleep apnea was not controlled for but sleep apnea is more likely to be undiagnosed in women – which could create a sample with women who have more severe cases. Other studies have also found that women may be more likely to be diagnosed with long COVID and seek health care for the condition.Long COVID is an umbrella term for one or more symptoms that people can experience for weeks months or years after a COVID-19 infection. Multiple definitions were included in this The Centers for Disease Control and Prevention defines long COVID as symptoms that last for at least four weeks after infection while the World Health Organization defines long COVID as symptoms that persist for at least three months.Obstructive sleep apnea occurs when the upper airway becomes blocked during sleep which interrupts breathing. The condition affects about 1 in 8 adults but is often underdiagnosed.The study was funded by RECOVER (OT2HL161847) and received additional support from the National Center for Advancing Translational Sciences (UL1TR002494).HHS Long COVID Coordination This work is a part of the National Research Action Plan a broader government-wide effort in response to the Presidential Memorandum directing the Secretary for the Department of Health and Human Services to mount a full and effective response to long COVID. Led by Assistant Secretary for Health Admiral Rachel Levine the Plan and its companion Services and Supports for Longer-term Impacts of COVID-19 report lay the groundwork to advance progress in the prevention diagnosis treatment and provision of services for individuals experiencing long COVID.Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses an electronic health record-based analysis from the RECOVER initiative | SLEEP | Oxford Academic (oup.com)National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/obstructive-sleep-apnea-associated-increased-risks-long-covid

Adopting pediatric readiness standards improves survival in hospital emergency departments
Friday January 13 2023The National Pediatric Readiness Project was established to ensure that all emergency departments have the coordination of health care personnel procedures and medical equipment needed to care for ill and injured children. According to the projects checklist standards include specifications for physician and nurse certification patient assessment triage medication administration and trauma resuscitation and stabilization. In the current study researchers sought to determine if adopting the readiness standards would lower the death rate among children admitted to emergency departments for serious injury or illness. They ranked the emergency departments into four segments (quartiles) according to the extent they had implemented the readiness standards.Compared to children cared for in low-readiness departments children with injuries cared for in high-readiness departments had a 60% lower chance of dying in the hospital; and children with medical illness had a 76% lower chance of dying while they were in the hospital. Similarly among roughly 545 000 children in six states injured children in the highest quartile had a 41% lower chance of dying within a year and children with medical issues had a 66% lower chance of dying within a year compared to children cared for in hospitals in the lowest readiness quartile.A previous study by the authors found that adopting the readiness centers at trauma centers improved the survival of children with serious injuries.Cinnamon Dixon D.O. M.P.H a medical officer in the NICHD Pediatric Trauma and Critical Illness Branch is available for comment.Newgard CD. Emergency department pediatric readiness and short- and long-term mortality among children receiving emergency care. JAMA Network Open. 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/adopting-pediatric-readiness-standards-improves-survival-hospital-emergency-departments

Vigorous exercise not tied to increased risk of adverse events in rare heart condition
Wednesday May 17 2023“Based on these data were learning that we dont need to universally restrict HCM patients from participating in vigorous exercise something thats so important to all of us ” said Rachel Lampert M.D. a professor of medicine at Yale School of Medicine in New Haven Connecticut one of the principal study authors and a practicing cardiologist who is an expert in arrhythmias in HCM.“Individuals with this condition should talk to a healthcare provider with expertise in HCM about getting back on the field back in the pool and back on the court if thats what they want to do ” Lampert added. “Getting an expert evaluation is key to determining degree of risk for all HCM patients and critical before going back to play.”HCM can make it harder for the heart to pump blood because the thickened ventricles (the lower chambers of the heart) become too stiff. This can cause some people to experience shortness of breath chest pain fatigue and more seriously a life-threatening irregular heartbeat known as arrhythmia. In rare cases HCM can cause sudden death. The condition is typically managed with medications or the use of surgically implanted devices such as an implantable cardioverter-defibrillator (ICD) which can detect an arrhythmia.Recommendations to restrict all exercise for most people with the disease have been based mainly on an abundance of caution in the absence of specific data. Large detailed multiyear studies on the health risks of exercise in people diagnosed with HCM have been lacking until now.For the study the researchers recruited 1 660 people who either had HCM or the gene for HCM but had not yet manifested the disease (8% of the total). They ranged from ages 8 to 60 and were recruited from 42 high-volume HCM medical centers in the United States and other countries including the United Kingdom Canada Australia and New Zealand. About 60% of participants were male. The study excluded people who could not exercise for established medical reasons such as those awaiting heart transplantation or with severe asthma.The participants were divided according to self-reported exercise levels based on a physical activity questionnaire used in research studies. About 15% of the participants reported being sedentary 43% said they did moderate exercise such as brisk walking and 42% said they did vigorous exercise such as running or fast swimming. The researchers then followed the groups for about three years and looked at the occurrence of four main cardiovascular events during that period sudden deaths resuscitated sudden cardiac arrests arrhythmic syncope (which can include fainting or passing out) and appropriate ICD shocks.To simplify these measurement outcomes the researchers used a statistical formula that measured a composite of these four events. The researchers found that 77 participants or 1.5% per year who reported exercising vigorously died or had severe cardiac events – the same percentage as those who exercised moderately or described themselves as sedentary. The outcome was similar for competitive exercisers (39% of the vigorous group) and for a subgroup of 42 young people who participated in interscholastic competitive sports such as baseball track soccer and basketball.“This finding is significant and provides a measure of reassurance that exercise may be safe for persons with HCM ” said Patrice Desvigne-Nickens M.D. a medical officer in the Heart Failure and Arrhythmias Branch in NHLBIs Division of Cardiovascular Sciences. “However we stress that individuals with the condition should not be exercising until theyve first had an evaluation by a provider with expertise in HCM about their overall risk of sudden cardiac death. It is important to know that all patients with HCM could potentially be at risk for sudden death.”This study was supported by grant 1R01HL125918 from NHLBI. Preliminary results of the study were previously described during a meeting of the American College of Cardiology in March.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Vigorous Exercise in Patients with Hypertrophic Cardiomyopathy Results of the Prospective Observational Multinational “Lifestyle and Exercise in HCM” (LIVE-HCM) Study. JAMA Cardiology. doi 10.1001/jamacardio.2023.1042National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/vigorous-exercise-not-tied-increased-risk-adverse-events-rare-heart-condition

Marburg vaccine shows promising results in first-in-human study
Monday January 30 2023This first-in-human Phase 1 study tested an experimental MARV vaccine candidate known as cAd3-Marburg which was developed at NIAIDs Vaccine Research Center (VRC). This vaccine uses a modified chimpanzee adenovirus called cAd3 which can no longer replicate or infect cells and displays a glycoprotein found on the surface of MARV to induce immune responses against the virus. The cAd3 vaccine platform demonstrated a good safety profile in prior clinical trials when used in investigational Ebola virus and Sudan virus vaccines developed by the VRC.MARV a filovirus in the same family as Ebola virus causes a rapidly progressive febrile illness that leads to shock and death in a large proportion of infected individuals. Many scientists think that MARV disease outbreaks in humans begin by when the virus makes the jump from its primary animal host which is likely to be certain chronically infected bats in sub-Saharan Africa. The symptoms of MARV disease are akin to those seen with Ebola virus disease and can include fever headache chills rash abdominal pain vomiting and diarrhea. As the disease progresses patients may suffer from multiple organ dysfunction delirium and significant bleeding from the gastrointestinal tract or other sites that may result in death. No approved vaccines or specific therapies are available for MARV disease aside from supportive care. While some experimental vaccines have previously been tested none have proven to be both highly effective and to provide durable protection. In areas of Africa where a vaccine for Marburg is most needed a single-dose vaccine that could protect recipients over a long period of time would be a crucial part of quelling outbreaks.In this study 40 healthy adult volunteers were enrolled at the Walter Reed Army Institute of Research Clinical Trials Center in Silver Spring Maryland. They received a single dose of either a low dose of the vaccine (1x1010 particle units) or a higher dose (1x1011 particle units). For safety the volunteers were enrolled in a dose-escalation plan. Three participants received the lower dose. Then when they did not exhibit severe adverse reactions after the first seven days the trial proceeded to enroll the remaining 17 volunteers. The same procedure was also used for the higher dose group. Volunteers were monitored for adverse reactions to the investigational vaccine and evaluated at regular intervals for 48 weeks to track their immune responses.The trials safety results were encouraging There were no serious adverse events and the experimental vaccine was well-tolerated. One participant in the higher dose group developed a fever following vaccination but it resolved by the following day. In addition the investigational vaccine appeared to induce strong long-lasting immunity to the MARV glycoprotein 95% of participants in the trial exhibited a robust antibody response after vaccination and 70% maintained that response for more than 48 weeks.Plans are in place to conduct further trials of the cAd3-Marburg vaccine in Ghana Kenya Uganda and the United States. If additional data supports the promising results seen in the Phase 1 trial the cAd3-Marburg virus vaccine could someday be used in emergency responses to MARV outbreaks.M Hamer et al. Safety tolerability and immunogenicity of the Marburg chimpanzee adenovirus vector vaccine (cAd3-Marburg) in healthy adults a phase 1 open-label dose-escalation trial. The Lancet DOI 10.1016/S0140-6736(22)02400-X (2023).Lesia Dropulic M.D. chief of the Clinical Trials Program at NIAIDs Vaccine Research Center is available for comment.To schedule interviews please contact Elizabeth Deatrick (301) 402-1663 NIAIDNews@niaid.nih.gov.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/marburg-vaccine-shows-promising-results-first-human-study

Biomarker pattern found in kids with COVID 19-linked inflammatory syndrome
Tuesday April 25 2023To conduct the study researchers analyzed 416 blood samples from 237 patients. Their analysis enabled them to distinguish between patients with MIS-C and COVID-19. They believe their findings could lead to the development of tests that allow clinicians to distinguish between MIS-C and other conditions involving widespread inflammation such as Kawasaki disease septic shock and severe COVID-19 and to the development of more appropriate treatments for each.A previous study of children and adolescents who received a COVID-19 vaccination following MIS-C found that there were no reports of serious complications including myocarditis or MIS-C reoccurrence after the injection. Everyone should stay up to date with COVID-19 vaccines for their age group as the U.S. Centers for Disease Control and Prevention recommends regardless of whether they have been infected with the virus.Sai Majji Ph.D. of the NICHD Maternal and Pediatric Infectious Diseases Branch is available for comment.Loy CJ et al. Nucleic acid biomarkers of immune response and cell and tissue damage in children with COVID-19 and MIS-C. Cell Reports Medicine.2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/biomarker-pattern-found-kids-covid-19-linked-inflammatory-syndrome

Researchers study enhanced genetic animal model of Down syndrome
Tuesday March 14 2023New mouse model may inform potential therapeutic options for Down syndrome.Scientists found that the new mouse model known as Ts66Yah had memory difficulties and behavior traits but the symptoms were not as severe as seen with the previous mouse model. Scientists often use different strains of mice as animal models to study human diseases because most genes in humans have similar counterparts in mice.About 6 000 newborns are diagnosed with Down syndrome each year in the United States. In most cases these babies have a third copy of chromosome 21. An additional chromosome 21 adds an extra copy of over 200 protein-coding genes to that persons genome which causes difficulties with learning speech and motor skills.A previous mouse model known as Ts65Dn has been considered the standard for Down syndrome research used in preclinical studies for nearly 30 years. Along with some successful cognitive treatments such as a recent hormone-based cognitive treatment some other treatments that were effective in the mouse model were not as effective in humans.Importantly the previous mouse models genome contains 45 extra genes that are irrelevant to human Down syndrome a byproduct of how the model was developed. Humans and mice have very similar genomes but the chromosomes that make up those genomes do not precisely align across those two species. For example many of the genes found on human chromosome 21 are found on mouse chromosomes 16 and 17. The previous mouse model has an additional region of mouse chromosome 17 that contains 45 extra genes not found on human chromosome 21. How these 45 extra genes affect the brain and behavior of the previous Ts65Dn mice has not been investigated until now.To create an enhanced mouse model of Down syndrome researchers at the University of Strasbourg France removed these extra 45 genes using CRISPR gene-editing technology. Dr. Bianchis group then compared the two mouse models and found that the extra 45 genes in the previous mouse model were affecting brain development and contributed to more severe difficulties with motor skills communication and memory.“There are considerable effects of these extra genes on mouse brain development and behavior ” said Faycal Guedj Ph.D. staff scientist in NHGRIs Center for Precision Health Research and first author of the study. “What was previously thought as the best mouse model of Down syndrome has traits derived from genes that are not relevant to human chromosome 21.”Researchers at the Center for Precision Health Research aim to use cutting-edge genomics tools to foster next-generation healthcare. With this new and improved mouse model Dr. Bianchis group hopes to develop more precise treatments for improving cognition with the goal of independent living skills in people with Down syndrome."The possibility of treating intellectual disabilities in the context of Down syndrome goes to the core of changing conceptions about the nature of disability its medical and clinical aspects and what we often pejoratively consider normal and desirable in the context of medical care and in society ” underscores Christopher R. Donohue Ph.D. NHGRI senior historian. “As cognitive treatments based on genetic models become more feasible in the future researchers in conversation with disability ethicists those with Down syndrome and other healthcare professionals should carefully weigh potential benefits versus drawbacks including contributing to ableism in medicine and other forms of stigma.”National Human Genome Research Institute (NHGRI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/researchers-study-enhanced-genetic-animal-model-down-syndrome

Screening tool aims to help doctors diagnose more people with COPD
Tuesday February 14 2023A new tool shows promise in helping primary care physicians identify adults with undiagnosed chronic obstructive pulmonary disease (COPD) according to research published in JAMA. More than 15 million Americans have been diagnosed with COPD a leading cause of death in the United States and experts predict millions more have it but dont know it.“The goal with trying to find COPD is to treat it earlier which will help make patients feel better and hopefully prevent their disease from progressing ” said Fernando J. Martinez M.D. a principal investigator of the study and chief of the Pulmonary and Critical Care Medicine division at Weill Cornell Medicine New York City.Conducted at seven U.S. clinical research network centers from October 2018 to April 2022 the trial involved 4 325 adults ages 45-80. By the end of the study researchers discovered that 110 participants 2.5% of the study sample had moderate to severe forms of COPD. CAPTURE identified 53 or 48% of these cases. However it provided false positives for 479 participants 11% who did not have COPD. All participants received COPD testing which is how researchers assessed the tools effectiveness.While the researchers said they are studying ways to improve CAPTUREs accuracy they emphasized the goal of the screening criteria is not to diagnose COPD but to identify patients who would benefit from COPD testing. The gold standard for diagnosing the condition is through spirometry a breathing test.Common COPD symptoms include coughing shortness of breath wheezing or whistling in the chest and chest tightness or heaviness. People with more advanced cases may experience limitations with regular activities due to increased risks for lung infections like the flu or pneumonia which can cause COPD flare-ups or exacerbations. Treatment options include different types of inhalers pulmonary rehabilitation and support with quitting smoking.As part of CAPTURE screening which is already used by some physicians patients answer five questions which assess their breathing and exposure to chemicals and air pollution. Those with medium scores of 2-4 indicative of moderate breathing issues take an in-office breathing test to measure their peak expiratory flow rate (PEFR) or the force of exhalation or lung function. Adults with PEFR scores of less than 250 L/min for women and less than 350 L/min for men get COPD testing. Those with higher questionnaire scores of 5-6 skip the PEFR test and automatically get COPD testing.One benefit of the CAPTURE screening the researchers said is that it gives doctors information to further assess patients with respiratory symptoms. Doctors tend to underuse spirometry – only about a third of COPD assessments include it – in part because some find the tests difficult to integrate into shorter primary care office visits.“CAPTURE was designed to be easy for physicians to use ” said Antonello Punturieri M.D. Ph.D. program director of NHLBIs Chronic Obstructive Pulmonary Disease/Environment Program. “The screening is simple takes less than a minute and helps identify adults with trouble breathing who should be evaluated further.”Based on the researchers analysis 1 in 81 CAPTURE screenings would identify an adult with treatable but previously undiagnosed COPD. Researchers are currently studying if making minor changes like altering questions or adding others might improve the tools accuracy so that even more patients with COPD can be identified. Additional studies are also needed to see if use of CAPTURE will improve patient health outcomes.“The study shows that there is a high degree of respiratory burden in primary care and physicians need to ask about it and do the appropriate testing to determine if symptoms are driven by COPD or another process so that patients can get the right treatment ” said MeiLan K. Han M.D. a principal investigator of the study and a professor of medicine in the Division of Pulmonary and Critical Care at the University of Michigan Ann Arbor.In the meantime larger studies are underway to assess CAPTURE and how physicians use the tool in practice. Results are expected to be available later this year. The research was partially funded by NHLBI through grant 1R01HL136682.To learn more about COPD and lung health visit https //www.nhlbi.nih.gov/BreatheBetter.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit https //www.nhlbi.nih.gov.Martinez FJ Han MK Lopez C et al. Discriminate accuracy of the CAPTURE tool for identifying chronic obstructive pulmonary disease in US primary care settings. JAMA. 2023; doi 10.1001/jama.2023.0128.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/screening-tool-aims-help-doctors-diagnose-more-people-copd

COVID-19 vaccine for children after MIS-C appears safe
Tuesday January 3 2023The multicenter observational study the largest of its kind to examine COVID vaccination in this group helps resolve a lingering question about whether the COVID vaccine can increase the risk of health problems in young people who have had MIS-C a rare and potentially fatal immunological reaction that can occur following infection with SARS-CoV-2 the virus that causes COVID-19.MIS-C is a poorly understood condition that affects 1 in about 3 000 to 4 000 children and adolescents who had COVID-19 according to the Centers for Disease Control and Prevention (CDC). It occurs a few weeks after COVID infection and can lead to organ failure. Symptoms can range from stomach pain fever and rash to inflammation of the heart muscle a serious condition called myocarditis. The exact causes of MIS-C are unknown but medications can be given to decrease the inflammation that can damage organs.Some families and healthcare professionals have questioned whether COVID vaccines could lead to more serious adverse reactions in those with a history of MIS-C including a recurrence of the disease but data on this topic were lacking.The cross-sectional study included 22 medical centers (21 in the United States and 1 in Canada) participating in the NHLBIs Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC) study. It enrolled 385 patients aged 5 years or older with prior MIS-C who were eligible for COVID-19 vaccination. Of this group 185 (48.1%) received at least one vaccine dose. The median age was 12.2 years and 73.5% were male. The participants were racially diverse – 24.3% were Black 31.9% were Hispanic and 28.6% were white. The median length of time from their MIS-C diagnosis to their first vaccine dose was 9 months.Of those who received a COVID vaccination following MIS-C mild adverse reactions – mostly arm soreness and fatigue – occurred in 49% of them similar to the general population. There were no reports of serious complications including myocarditis or recurrence of MIS-C the researchers said. “We are very reassured by the results and this safety data should be comforting to families and healthcare professionals when considering and recommending vaccination ” said study co-leader Matthew D. Elias M.D. a pediatric cardiologist at Childrens Hospital of Philadelphia and clinical assistant professor of pediatrics at the University of Pennsylvania Philadelphia. Audrey Dionne M.D. a pediatric cardiologist at Boston Childrens Hospital and assistant professor of pediatrics at Harvard Medical School Boston also served as the studys co-leader. The researchers have routinely treated children with MIS-C throughout the pandemic.Dionne added that the findings provide support for the CDCs recommendation that patients with a history of MIS-C receive a COVID vaccine at least 90 days after diagnosis and that it is safe to do so.To date more than 9 000 patients have been diagnosed with MIS-C in the United States and 74 have died according to data from the CDC (https //covid.cdc.gov/covid-data-tracker/#mis-national-surveillance). However the disease appears to be on the decline according to studies by others.“A big part of that decline is that COVID vaccination has been protective against this rare condition in those who have received it ” Dionne said.Research reported in this study was funded by the NHLBIs MUSIC study which was supported by grants HL135680 HL135685 HL135683 HL135689 HL135646 HL135665 HL135678 HL135682 HL135666 HL135691 and HL068270. The study uses the research infrastructure of the Pediatric Heart Network a pediatric cardiology research consortium funded by the NHLBI and its data coordinating center HealthCore Inc.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Examination of adverse reactions after COVID-19 vaccination among patients with a history of multisystem inflammatory syndrome in children. JAMA Network Open. DOI 10.1001/jamanetworkopen.2022.48987National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/covid-19-vaccine-children-after-mis-c-appears-safe
Potential treatment for rare autoimmune disorder adapted from CAR-T therapy
Thursday June 22 2023“Repurposing a groundbreaking therapy such as CAR-T to potentially treat a neurological disorder shows the versatility of immunotherapies in instances where there are limited to no treatment options ” said Emily Caporello Ph.D. director of the NINDS Small Business Program.Myasthenia gravis is a chronic autoimmune disorder most often caused when the bodys immune system attacks a protein found where nerve cells communicate with muscles. The disease is marked by muscle weakness that worsens after periods of activity and can improve somewhat after rest. Current treatments focus on controlling symptoms primarily muscle weakness.In the study 14 people with generalized myasthenia gravis received varying doses of a modified form of CAR-T therapy known as Descartes-08 targeting the cells responsible for producing myasthenia gravis-causing antibodies. The ideal dosage was determined to be once weekly for six weeks. Early data on the effectiveness of the treatment are promising but additional clinical studies are needed to evaluate the therapys efficacy. Three patients receiving Descartes-08 showed complete or near-complete elimination of their symptoms which continued six months following treatment. Two others no longer required chronic intravenous immunoglobulin treatment which is currently used in some severe MG cases.“What we saw were deep durable responses to Decartes-08 that persisted for at least six months following treatment ” said Murat V. Kalayoglu M.D. Ph.D. president and CEO of Cartesian Therapeutics. “We have now begun a larger randomized placebo-controlled study which is the first of its kind for an engineered adoptive cell therapy.”CAR-T therapy involves taking a patients T-cells — a key part of the immune system that can recognize and destroy invading pathogens—and reprogramming them to attack a specific target. In the case of blood cancers the new target is the cancer itself. For myasthenia gravis the target is the corrupted cells that make the damaging antibodies.Many immunotherapies including CAR-T carry the risk of serious side effects that while tolerable in the cases of advanced cancer prohibit its use in more chronic conditions such as myasthenia gravis. Normally T-cells are reprogrammed with DNA which persists within the cells and is copied every time the cells divide. This can lead to an amplified effect and serious side effects.To avoid this side effect Descartes-08 uses messenger RNA (mRNA) which does not duplicate when cells divide instead of DNA to reprogram T-cells. The result is a short course of treatment that is given multiple times instead of the single-dose regimen normally used in DNA programmed CAR-T therapy. The primary aim of this trial was to determine the ideal dosing for Descartes-08 that effectively reduces muscle weakness symptoms with minimal side effects.Descartes-08 therapy is now being tested in a larger clinical trial to determine its ability to reduce myasthenia gravis symptoms. Importantly this trial will also include a placebo group which is an important control to confirm that any observed improvement is due to the treatment rather than any unrelated effects.This trial was supported by a grant from the NINDS Small Business Program (NS115426-01A1) which is designed to encourage research and development that can lead to commercial therapeutic products.. The clinical trial reported in this study and the ongoing phase 2 trial are registered on ClinicalTrials.gov (NCT04146051).Granit V. Benatar M. Kurtoglu M. et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001) a prospective multicentre open-label non-randomised phase 1b/2a study. Lancet Neurol.June 21 2023. DOI 10.1016/PIIS1474-4422(23)00194-1This page last reviewed on June 22 2023U.S. Department of Health and Human Services
https://www.nih.gov/news-events/potential-treatment-rare-autoimmune-disorder-adapted-car-t-therapy

Experimental HIV vaccine regimen safe but ineffective, study finds
Wednesday January 18 2023The clinical trial began in 2019 and involved 3 900 volunteers.An investigational HIV vaccine regimen tested among men who have sex with men (MSM) and transgender people was safe but did not provide protection against HIV acquisition an independent data and safety monitoring board (DSMB) has determined. The HPX3002/HVTN 706 or “Mosaico ” Phase 3 clinical trial began in 2019 and involved 3 900 volunteers ages 18 to 60 years in Europe North America and South America. Based on the DSMBs recommendation the study will be discontinued. Participants are being notified of the findings and further analyses of the study data are planned.The experimental vaccine regimen was developed by Janssen. It was based on “mosaic” immunogens—vaccine components featuring elements of multiple HIV subtypes—with the goal of inducing immune responses against a wide variety of global HIV strains. The investigational vaccine regimen consisted of four injections over a year of Ad26.Mos4.HIV. This vaccine candidate uses a common-cold virus (adenovirus serotype 26 or Ad26) to deliver the mosaic immunogens. The final two vaccinations were accompanied by a bivalent (two-component) HIV envelope protein formulation combining clade C gp140 and mosaic gp140 envelope proteins adjuvanted by aluminum phosphate to boost immune responses. All study vaccinations were completed in October 2022.In its scheduled data review the DSMB determined there were no safety issues with the experimental vaccine regimen. However the number of HIV infections were equivalent between the vaccine and placebo arms of the study. During the clinical trial all participants were offered comprehensive HIV prevention tools including pre-exposure prophylaxis or PrEP. Study staff ensured that participants who acquired HIV during the trial were promptly referred for medical care and treatment.The Mosaico findings track with developments in the Phase 2b “Imbokodo” (HPX2008/HVTN 705) clinical trial which was testing a similar HIV vaccine regimen in young women in sub-Saharan Africa. A DSMB determined in 2021 that the experimental vaccine regimen in that study was also safe but ineffective in protecting against HIV acquisition.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/experimental-hiv-vaccine-regimen-safe-ineffective-study-finds

Large study provides scientists with deeper insight into long COVID symptoms
Thursday May 25 2023“Americans living with long COVID want to understand what is happening with their bodies ” said ADM Rachel L. Levine M.D. Assistant Secretary for Health. “RECOVER as part of a broader government response in collaboration with academia industry public health institutions advocacy organizations and patients is making great strides toward improving our understanding of long COVID and its associated conditions.”Researchers examined data from 9 764 adults including 8 646 who had COVID-19 and 1 118 who did not have COVID-19. They assessed more than 30 symptoms across multiple body areas and organs and applied statistical analyses that identified 12 symptoms that most set apart those with and without long COVID post-exertional malaise fatigue brain fog dizziness gastrointestinal symptoms heart palpitations issues with sexual desire or capacity loss of smell or taste thirst chronic cough chest pain and abnormal movements.They then established a scoring system based on patient-reported symptoms. By assigning points to each of the 12 symptoms the team gave each patient a score based on symptom combinations. With these scores in hand researchers identified a meaningful threshold for identifying participants with long COVID. They also found that certain symptoms occurred together and defined four subgroups or “clusters” with a range of impacts on health.Based on a subset of 2 231 patients in this analysis who had a first COVID-19 infection on or after Dec. 1 2021 when the Omicron variant was circulating about 10% experienced long-term symptoms or long COVID after six months. The results are based on a survey of a highly diverse set of patients and are not final. Survey results will next be compared for accuracy against an array of lab tests and imaging.To date more than 100 million Americans have been infected with SARS-CoV-2 the virus that causes COVID-19. As of April the federal governments Household Pulse survey estimates that about 10% of adults infected with the virus continue to experience and suffer from the many symptoms termed together as long COVID. Patients and researchers have identified more than 200 symptoms associated with long COVID.“This study is an important step toward defining long COVID beyond any one individual symptom ” said study author Leora Horwitz M.D. director of the Center for Healthcare Innovation and Delivery Science and co-principal investigator for the RECOVER Clinical Science Core at NYU Langone Health. “This approach — which may evolve over time — will serve as a foundation for scientific discovery and treatment design.” The researchers explain studying the underlying biological mechanisms of long COVID is central to advancing informed interventions and identifying effective treatment strategies.In addition to establishing the scoring system the researchers found that participants who were unvaccinated or who had COVID-19 before the Omicron strain emerged in 2021 were more likely to have long COVID and more severe cases of long COVID. Further reinfections were also linked to higher long COVID frequency and severity compared to people who only had COVID-19 once.The ongoing RECOVER research serves as the foundation for planned clinical trials whose interventions are rooted in many of the symptoms outlined in this study. RECOVER clinical trials are expected to begin enrolling patient participants in 2023.HHS Long COVID Coordination This work is a part of the National Research Action Plan (opens pdf) a broader government-wide effort in response to the Presidential Memorandum directing the Secretary for the Department of Health and Human Services to mount a full and effective response to long COVID. Led by Assistant Secretary for Health Admiral Rachel Levine the Plan and its companion Services and Supports for Longer-term Impacts of COVID-19 (opens pdf) report lay the groundwork to advance progress in the prevention diagnosis treatment and provision of services for individuals experiencing long COVID.Thaweethai T Jolley SE Karlson EW et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA. Published online May 25 2023. doi 10.1001/jama.2023.8823On May 31 2023 a statistic in the seventh paragraph on the percentage of adults continuing to experience symptoms of COVID-19 was updated from 6% to 10%.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/large-study-provides-scientists-deeper-insight-into-long-covid-symptoms

Toxic protein linked to muscular dystrophy and arhinia
Friday February 17 2023FSHD type 2 (FSHD2) is an inherited form of muscular dystrophy that causes progressive muscle weakness. Arhinia is an extremely rare yet severe disorder that prevents the development of an external nose and the olfactory bulbs and tracts. Both diseases are caused by mutations in the SMCHD1 gene. In patients with FSHD2 there is overproduction of DUX4 which kills the muscle cells and this leads to the progressive weakening of the muscles.Shaws team found that the combination of the mutated SMCHD1 gene and an environmental modifier such as a virus may trigger the DUX4 toxic protein. This may be what causes arhinia to occur. Using stem cells created from patients with the two diseases the researchers conducted studies in cranial placode cells the cells that lead to the development of the bodys sensory organs such as the nose. As the placode cells started to form they began to produce the DUX4 protein which caused cell death.The researchers showed that DUX4 is responsible for cell death in placode cells as it is in muscle cells but they still do not understand why the nose cells do not die in muscular dystrophy or why the muscle cells are not dying in arhinia.“Now what we have to do is try to figure out the players acting downstream of DUX4 so we can block it from damaging the muscle cells or the nose precursors and hopefully find some new treatment options for patients suffering from these rare diseases ” said Shaw.Kaoru Inoue Hamed Bostan MaKenna R. Browne Owen F. Bevis Carl D. Bortner Steven A. Moore Aaron A. Stence Negin P. Martin Shih-Heng Chen Adam B. Burkholder Jian-Liang Li and Natalie D. Shaw. DUX4 double whammy the transcription factor that causes a rare muscular dystrophy also kills the precursors of the human nose. Science Advances. DOI 10.1126/sciadv.abq7744U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/toxic-protein-linked-muscular-dystrophy-arhinia

Men died of overdose at 2-3 times greater a rate than women in the U.S. in 2020-2021
Wednesday June 14 2023National data show need for more research into the diverse biological behavioral and social factors underlying differences in sex-based vulnerability to drug use.Men were significantly more vulnerable than women to overdose deaths involving opioid and stimulant drugs in 2020-2021 according to a new study analyzing death records data from across the United States. The study found that men had a 2–3 times greater rate of overdose mortality from opioids (like fentanyl and heroin) and psychostimulants (like methamphetamine and cocaine). While it has been known that men use drugs at higher rates than women the researchers found that this alone does not explain the gap in overdose deaths noting that biological behavioral and social factors likely combined to increase the mortality risk for men.“Though men and women are being exposed to the modern fentanyl-contaminated drug supply something is leading men to die at significantly higher rates. It may be that men use drugs more frequently or in greater doses which could increase their risk of death or there may be protective factors among women that reduce their risk of death compared to men ” said Nora Volkow M.D. director of NIDA and one of the co-authors on the study. “Understanding the biological behavioral and social factors that impact drug use and our bodies responses is critical to develop tailored tools to protect people from fatal overdose and other harms of drug use.”In 2021 nearly 107 000 people died of a drug overdose largely driven by potent illicit fentanyl which now contaminates the drug supply. Data have consistently shown that the rate of drug overdose deaths is significantly higher for men than women. In addition data suggest that men are more likely than women to use almost all types of illicit drugs. Building on these data researchers sought to determine the extent to which this known sex difference in overdose mortality varies by drug state and age and to investigate whether the increased rate of overdose death among men held true when controlling for higher rates of drug misuse among men compared to women.To do so researchers conducted a state-by-state analysis of nationally representative epidemiological data on overdose mortality among people aged 15–74 from 2020-2021 in the U.S. using the Centers for Disease Control and Preventions Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) platform. The scientists also used state-level nationally representative data from the National Surveys on Drug Use and Health (NSDUH) to estimate and control for rates of drug misuse (taking drugs in a way not recommended by a health care provider) among men compared to women. The NSDUH is conducted annually by the Substance Abuse and Mental Health Services Administration.For specific drugs and after controlling for the sex-specific rate of drug misuse the researchers found that the overall rates of drug overdose death by sex from 2020-2021 wereThe higher overdose death rate in men was observed across the lifespan (ages 15-74 overall) and was consistent across states even after accounting for other demographic factors such as household net worth. In addition when the authors analyzed the data by 10-year age groups they found that for overdose deaths involving synthetic opioids like fentanyl men had greater rates than women across each group within the entire 15-74 age range measured in the study. For the three other drug categories assessed men also had greater overdose mortality rates compared to women across the lifespan with few exceptions. Due to limited data for heroin the youngest and oldest age groups (age ranges 15-24 and 65-74) were excluded from analysis; for psychostimulants and cocaine the oldest age group (age range 65-74) was excluded from analysis.While researchers also found that men reported misusing drugs more than women the magnitude of difference recorded for overdose mortality between men and women was substantially greater than the difference of reported drug misuse. For example by comparing the data from CDC WONDER and NSDUH the researchers found that men had a 2.8 greater rate of cocaine overdose mortality compared to women though men only had a 1.9 greater rate of cocaine misuse compared to women.The authors hypothesize that it is a combination of biological (e.g. men may have a greater vulnerability to the toxicity of drugs than women) behavioral (e.g. men may use these drugs in a riskier way than women) as well as other social- and gender-related factors.“These data emphasize the importance of looking at the differences between men and women in a multilayered way ” said Eduardo R. Butelman Ph.D. assistant professor of psychiatry at the Icahn School of Medicine at Mount Sinai and a lead author on the study. “Moving forward it will be important for researchers to continue to investigate how biology social factors and behaviors intersect with sex and gender factors and how all of these can impact addictive drug misuse and overdose deaths.”For more information on substance and mental health treatment programs in your area call the free and confidential National Helpline 1-800-662-HELP (4357) or visit www.FindTreatment.gov.E. Butelman et al. Overdose mortality rates for opioids and stimulant drugs are substantially higher in men than in women State-level analysis. Neuropsychopharmacology. DOI 10.1038/s41386-023-01601-8 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/men-died-overdose-2-3-times-greater-rate-women-us-2020-2021

Only 1 in 4 adolescent treatment facilities offer buprenorphine for opioid use disorder
Tuesday June 13 2023There has been a dramatic rise in overdose deaths among young people aged 14-18 in recent years likely driven by illicit counterfeit pills containing fentanyl. For those with opioid use disorder medications are the most effective treatment options for preventing both return to opioid use and overdose deaths. Buprenorphine is the only medication for opioid use disorder that is approved by the U.S. Food and Drug Administration for use in people aged 16-18. Although buprenorphine is not approved for use among people under the age of 16 in the U.S. some professional medical societies recommend that buprenorphine be considered as a treatment option for opioid use disorder in younger people.“It is tragic to see that young people with opioid use disorder are unable to access buprenorphine in most treatment facilities despite this medication being the standard of care for people aged 16 and older” said Nora Volkow M.D. director of NIDA. “Residential treatment facilities provide an opportunity to reach young people with a range of evidence-based supports at a pivotal time in their lives and it is crucial that buprenorphine is made available as one of those options.”Residential treatment facilities are part of the continuum of care for adolescents with opioid use disorder. However little is known about specific evidence-based treatment options offered to young people at these facilities including medications for opioid use disorder. To address this gap researchers at OHSU sought to determine how many adolescent treatment centers in the U.S. were offering buprenorphine to treat opioid use disorder.Using the FindTreatment.gov database which is maintained by the Substance Abuse and Mental Health Services Administration (SAMHSA) the researchers identified a list of 354 centers across the U.S. that offered treatment for “substance use ” in a “residential/24-hour residential” service setting and for “children/adolescents” (defined as people aged 17 and younger) to include in the analysis.Researchers called these facilities to inquire about treatment and services offered as potential users of these services for a 16-year-old with a recent non-fatal fentanyl overdose. Between October and December 2022 the study team called the facilities in a random order and confirmed that 160 (45%) of these facilities provided residential treatment to patients under the age of 18.Of the 160 residential addiction treatment facilities found to provide treatment to young patients the researchers found that 39 facilities (24%) said that they offered buprenorphine to patients aged 16 or older including through partnership with outside prescribing clinicians though specific parameters for offering buprenorphine varied by site. For instance only 20 facilities (12.5%) said that they offered buprenorphine for ongoing treatment. 12 facilities (7.5%) said that they offered buprenorphine to adolescents under 16 years of age.Among the other 121 facilities that did not offer buprenorphine to adolescents or werent sure 57 (47%) indicated that adolescents prescribed buprenorphine by their own clinician could stay on it at least temporarily although some stated they would discontinue it before discharge. And 27 (22%) required that adolescents were not taking buprenorphine in order to be admitted for residential treatment.Based on these findings the average person would need to call nine facilities on the SAMHSA list to find one that offered buprenorphine. To find one for an adolescent under 16 they would need to call 29 facilities.“These residential treatment centers see some of the most vulnerable adolescents in our communities ” said lead author Caroline King M.D. Ph.D. who completed this research as a student in the OHSU School of Medicine. “We need to support these centers to make evidence-based care the norm.”“Buprenorphine is the one medication thats approved for use in adolescents and its underused in facilities taking care of kids with the most severe opioid use disorder ” said co-author Todd Korthuis M.D. M.P.H. head of addiction medicine at OHSU. “Its a big issue but its something that we can change by supporting these treatment centers with education and technical assistance about buprenorphine better funding to staff these centers and by letting the public know that buprenorphine is necessary treatment in healing brains.”For more information on substance and mental health treatment programs in your area call the free and confidential National Helpline 1-800-662-HELP (4357) or visit www.FindTreatment.gov.C King et al. Treatments used among adolescent residential addiction treatment facilities in the United States 2022. JAMA. DOI 10.1001/jama.2023.6266 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/only-1-4-adolescent-treatment-facilities-offer-buprenorphine-opioid-use-disorder

Scientists release a new human “pangenome” reference
Wednesday May 10 2023More complete and sophisticated collection of genome sequences captures significantly more human diversity.The new “pangenome” reference includes genome sequences of 47 people with the researchers pursuing the goal of increasing that number to 350 by mid-2024. With each person carrying a paired set of chromosomes the current reference actually includes 94 distinct genome sequences with a goal of reaching 700 distinct genome sequences by the completion of the project.The work appearing in the journal Nature is one of several papers published today by consortium members.A genome is the set of DNA instructions that helps each living creature develop and function. Genome sequences differ slightly among individuals. In the case of humans any two peoples genomes are on average more than 99% identical. The small differences contribute to each persons uniqueness and can provide insights about their health helping to diagnose disease predict outcomes and guide medical treatments.To understand these genomic differences scientists create reference human genome sequences for use as a “standard” — a digital amalgamation of human genome sequences that can be used as a comparison to align assemble and study other human genome sequences.The original reference human genome sequence is nearly 20 years old and has been regularly updated as technology advances and researchers fix errors and discover more regions of the human genome. However it is fundamentally limited in its representation of the diversity of the human species as it consists of genomes from only about 20 people and most of the reference sequence is from only one person.“Everyone has a unique genome so using a single reference genome sequence for every person can lead to inequities in genomic analyses ” said Adam Phillippy Ph.D. senior investigator in the Computational and Statistical Genomics Branch within NHGRIs Intramural Research Program and a co-author of the main study. “For example predicting a genetic disease might not work as well for someone whose genome is more different from the reference genome.”Using advanced computational techniques to align the various genome sequences the researchers constructed a new human pangenome reference with each assembly in the pangenome covering more than 99% of the expected sequence with more than 99% accuracy. It also builds upon the previous reference genome sequence adding over 100 million new bases or “letters” in DNA. While the previous reference genome sequence was single and linear the new pangenome represents many different versions of the human genome sequence at the same time. This gives researchers a wider range of options for using the pangenome in analyzing other human genome sequences.“By using the pangenome reference we can more accurately identify larger genomic variants called structural variants ” said Mobin Asri a Ph.D. student at the University of California Santa Cruz and co-first author of the paper. “We are able to find variants that were not identified using previous methods that depend on linear reference sequences."Structural variants can involve thousands of bases. Until now researchers have been unable to identify the majority of structural variants that exist in each human genome using short-read sequencing due to the bias of using a single reference sequence.“The human pangenome reference will enable us to represent tens of thousands of novel genomic variants in regions of the genome that were previously inaccessible ” said Wen-Wei Liao a Ph.D. student at Yale University and co-first author of the paper. “With a pangenome reference we can accelerate clinical research by improving our understanding of the link between genes and disease traits.”The total cost of supporting the work of the Human Pangenome Reference Consortium is projected to be about $40 million over five years which includes efforts to create the human pangenome reference improve DNA sequencing technology operate a coordinating center conduct outreach and create resources for the research community to use the pangenome reference.“Basic researchers and clinicians who use genomics need access to a reference sequence that reflects the remarkable diversity of the human population. This will help make the reference useful for all people thereby helping to reduce the chances of propagating health disparities ” said Eric Green M.D. Ph.D. NHGRI director. “Creating and enhancing a human pangenome reference aligns with NHGRIs goal of striving for global diversity in all aspects of genomics research which is crucial to advance genomic knowledge and implement genomic medicine in an equitable way.”In line with this effort the Human Pangenome Reference Consortium includes an embedded ethics group that is working to anticipate challenging issues and help guide informed consent prioritize the study of different samples explore possible regulatory issues pertaining to clinical adoption and work with international and Indigenous communities to incorporate their genome sequences in these broader efforts.Institutions involved in the Human Pangenome Reference Consortium may be found on the projects main page.National Human Genome Research Institute (NHGRI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/scientists-release-new-human-pangenome-reference

Pragmatica-Lung Study, a streamlined model for future cancer clinical trials, begins enrolling patients
Wednesday April 12 2023“This study is designed to eliminate potential barriers to enrollment and provides a model for increasing diversity and enrollment in clinical trials ” said Monica M. Bertagnolli M.D. director of NCI. “Pragmatica-Lung with its critical public and private partnerships reflects the innovative approaches NCI is taking to achieve the Cancer Moonshot℠ goals including reducing the cancer death rate by 50% within the next 25 years.”The trial will evaluate whether a combination of two FDA-approved medications ramucirumab (Cyramza manufactured by Eli Lilly and Company) and pembrolizumab (Keytruda manufactured by Merck) improves overall survival (how long people live) over standard treatment in people with advanced NSCLC whose disease has progressed after previous treatment with immunotherapy and chemotherapy.“These trials will make it easier for physicians who do not work in big academic medical centers to enroll their patients resulting in participation by more diverse populations ” said James H. Doroshow M.D. director of NCIs Division of Cancer Treatment and Diagnosis. “Making trials more accessible while upholding rigorous scientific and safety standards means that more health care practitioners and patients will have an opportunity to participate.”The trial will enroll up to 700 participants from around the United States. Adults ages 18 and older with stage 4 or recurrent NSCLC who were previously treated with immune checkpoint inhibitors (a type of immunotherapy) and chemotherapy will be randomly assigned to receive either ramucirumab plus pembrolizumab or standard treatment. The study will look primarily at how long patients in the two groups live and is expected to complete enrollment by the end of 2025.Pragmatica-Lung seeks to confirm the encouraging results of a randomized phase 2 clinical trial (S1800A) conducted as part of the Lung Cancer Master Protocol (Lung-MAP) the first lung cancer precision medicine trial supported by NCI. That trial involved 136 patients with advanced NSCLC who had been previously treated with chemotherapy and immunotherapy and it found evidence that the combination of ramucirumab plus pembrolizumab extended survival compared with standard treatment. Ramucirumab is a targeted drug that works by preventing new blood vessels from growing and pembrolizumab is an immunotherapy that helps the bodys immune system attack the cancer.The pragmatic approach is most appropriate for trials in which the drugs being studied have already been approved and their side effects are well understood Dr. Doroshow said.NCI collaborators for the Pragmatica-Lung Study include the SWOG Cancer Research Network which designed and is leading the trial in collaboration with the Alliance for Clinical Trials in Oncology.The study will be conducted with the participation of SWOG Alliance and two other U.S. NCI National Clinical Trials Network (NCTN) groups that focus on cancer in adults ECOG-ACRIN Cancer Research Group and NRG Oncology.Together these four NCTN groups represent more than 1 600 cancer treatment institutions in the United States and doctors from any of these sites can enroll patients on the trial.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/pragmatica-lung-study-streamlined-model-future-cancer-clinical-trials-begins-enrolling-patients

Forgoing one food treats eosinophilic esophagitis as well as excluding six
Monday February 27 2023EoE is a chronic disease characterized by an overabundance of white blood cells called eosinophils in the esophagus. Allergic inflammation due to food drives the disease by damaging the esophagus and preventing it from working properly. For people with EoE swallowing even small amounts of food can be a painful and stressful choking experience. About 160 000 people in the United States are living with EoE.Excluding certain foods from the diet has been a cornerstone of EoE treatment. During the early 2000s researchers found that eliminating six common food triggers of esophageal injury—milk egg wheat soy fish and nuts—substantially reduced signs and symptoms of EoE. This six-food elimination diet (6FED) became a common approach to managing the disease.In recent years scientists have conducted small non-randomized studies of removing one to four of the most common food antigens from the diet to treat EoE with some success. However the relative risks and benefits of eliminating many foods versus a few foods at the start of diet-based therapy remained unclear.The investigators found that 34% of participants on 6FED and 40% of participants on 1FED achieved remission after six weeks of diet therapy a difference that was not statistically significant. The two diets also had a similar impact across several other measures including reduction in EoE symptoms and effect on quality of life. Thus 1FED and 6FED were equally effective at treating EoE an unexpected finding.The researchers also discovered that nearly half of people who did not respond to 1FED attained remission after treatment with the more restrictive 6FED while more than 80% of the non-responders to 6FED achieved remission with oral steroids.Taken together the investigators conclude that 1FED is a reasonable first-line diet therapy option in adults with EoE and that effective therapies are available for people who do not achieve remission after 1FED or 6FED.KL Kliewer et al. One-food versus six-food elimination diet therapy for the treatment of eosinophilic oesophagitis a multicentre randomised open-label trial. The Lancet Gastroenterology & Hepatology DOI 10.1016/S2468-1253(23)00012-2 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/forgoing-one-food-treats-eosinophilic-esophagitis-well-excluding-six

New MRI method provides detailed view of the placenta during pregnancy
Wednesday February 1 2023The study team led by Yong Wang Ph.D. at Washington University in St. Louis and Alison Cahill M.D. at the University of Texas at Austin developed analysis methods for MRI scans that are routinely collected in hospitals and healthcare facilities. Importantly these types of MRI scans do not require contrast agents which are only used during pregnancy for limited circumstances. The teams machine learning method automatically processes MRI data to visualize separate placental compartments including the intervillous space (the area where maternal blood enters to provide nutrients and gas exchange) placental vessels and placental tissue. Unlike current MRI analysis methods which can only measure placental oxygen as an average across the entire organ the new method can characterize oxygen levels within these discrete compartments.The study team also used these region-specific measurements to look for differences between 22 study participants with healthy pregnancies and five participants who went on to develop pregnancy complications. While they did observe trends — highlighting the potential for this technique to detect specific pregnancy complications — further analysis is needed among larger groups of study participants.Overall the new method can serve as an objective quantitative method to assess placental health during pregnancy. With additional validation and refinement of this technique the method may be used by healthcare providers as a tool in the care of pregnant patients especially those at risk for pregnancy complications. Early monitoring of the placenta can lead to better detection and prevention of pregnancy complications including preterm birth fetal growth disorders and preeclampsia.David Weinberg Ph.D. Lead of the NICHD Human Placenta Project is available for interviews.To arrange an interview with Dr. Weinberg please e-mail nichdpress@mail.nih.gov or call 301-496-5133.Sun Z. et. al. Association of intraplacental oxygenation patterns on dual-contrast MRI with placental abnormality and fetal brain oxygenation. Ultrasound in Obstetrics & Gynecology DOI 10.1002/uog.24959 (2023)Journal cover image https //obgyn.onlinelibrary.wiley.com/doi/abs/10.1002/uog.24939U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/new-mri-method-provides-detailed-view-placenta-during-pregnancy
Good hydration linked to healthy aging
Tuesday January 3 2023The study expands on research the scientists published in March 2022 which found links between higher ranges of normal serum sodium levels and increased risks for heart failure. Both findings came from the Atherosclerosis Risk in Communities (ARIC) study which includes sub-studies involving thousands of Black and white adults from throughout the United States. The first ARIC sub-study started in 1987 and has helped researchers better understand risk factors for heart disease while shaping clinical guidelines for its treatment and prevention.For this latest analysis researchers assessed information study participants shared during five medical visits – the first two when they were in their 50s and the last when they were between ages 70-90. To allow for a fair comparison between how hydration correlated with health outcomes researchers excluded adults who had high levels of serum sodium at baseline check-ins or with underlying conditions like obesity that could affect serum sodium levels. They then evaluated how serum sodium levels correlated with biological aging which was assessed through 15 health markers. This included factors such as systolic blood pressure cholesterol and blood sugar which provided insight about how well each persons cardiovascular respiratory metabolic renal and immune system was functioning. They also adjusted for factors like age race biological sex smoking status and hypertension.They found that adults with higher levels of normal serum sodium – with normal ranges falling between 135-146 milliequivalents per liter (mEq/L) – were more likely to show signs of faster biological aging. This was based on indictors like metabolic and cardiovascular health lung function and inflammation. For example adults with serum sodium levels above 142 mEq/L had a 10-15% associated increased odds of being biologically older than their chronological age compared to ranges between 137-142 mEq/L while levels above 144 mEq/L correlated with a 50% increase. Likewise levels of 144.5-146 mEq/L were associated with a 21% increased risk of premature death compared to ranges between 137-142 mEq/L. Similarly adults with serum sodium levels above 142 mEq/L had up to a 64% increased associated risk for developing chronic diseases like heart failure stroke atrial fibrillation and peripheral artery disease as well as chronic lung disease diabetes and dementia. Conversely adults with serum sodium levels between 138-140 mEq/L had the lowest risk of developing chronic disease. The findings dont prove a causal effect the researchers noted. Randomized controlled trials are necessary to determine if optimal hydration can promote healthy aging prevent disease and lead to a longer life. However the associations can still inform clinical practice and guide personal health behavior.“People whose serum sodium is 142 mEq/L or higher would benefit from evaluation of their fluid intake ” Dmitrieva said. She noted that most people can safely increase their fluid intake to meet recommended levels which can be done with water as well as other fluids like juices or vegetables and fruits with a high water content. The National Academies of Medicine for example suggest that most women consume around 6-9 cups (1.5-2.2 liters) of fluids daily and for men 8-12 cups (2-3 liters).Others may need medical guidance due to underlying health conditions. “The goal is to ensure patients are taking in enough fluids while assessing factors like medications that may lead to fluid loss ” said Manfred Boehm M.D. a study author and director of the Laboratory of Cardiovascular Regenerative Medicine. “Doctors may also need to defer to a patients current treatment plan such as limiting fluid intake for heart failure.” The authors also cited research that finds about half of people worldwide dont meet recommendations for daily total water intake which often starts at 6 cups (1.5 liters).About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Dmitrieva NI Gagarin A Liu D et al. Middle-age high normal serum sodium as a risk factor for accelerated biological aging chronic diseases and premature mortality. eBioMedicine. 2023. doi 10.1016/j.ebiom.2022.104404.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/good-hydration-linked-healthy-aging
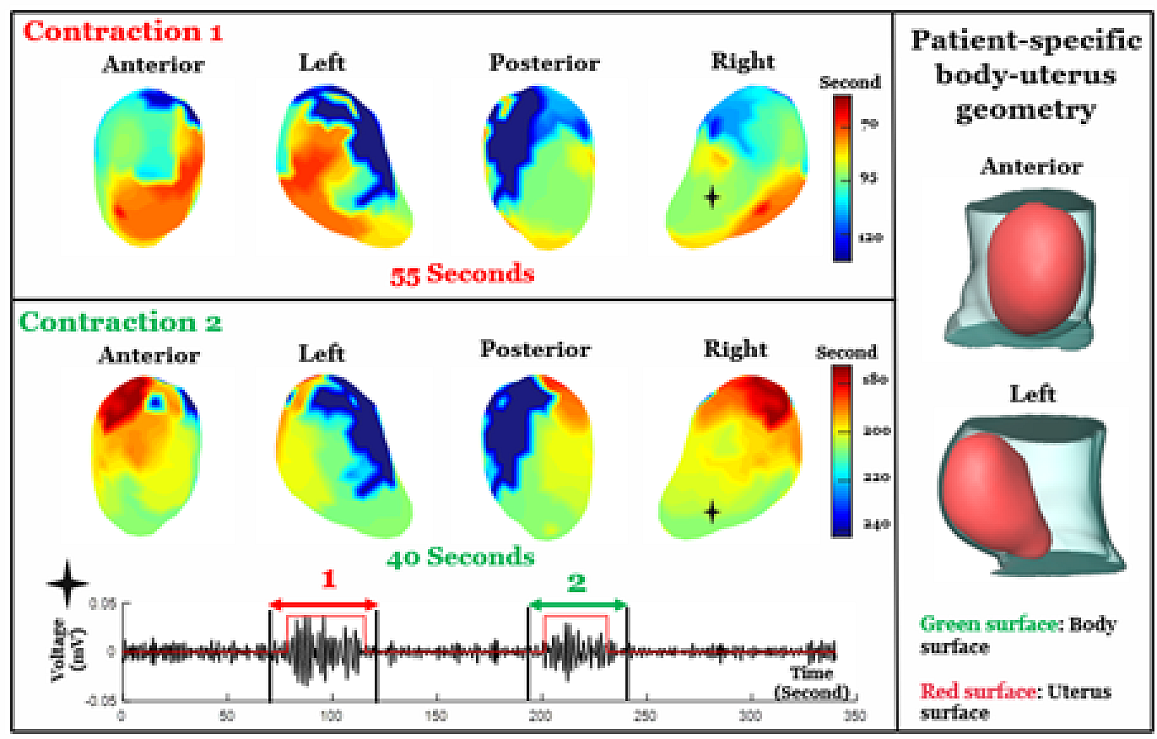
New, non-invasive imaging tool maps uterine contractions during labor
Tuesday March 14 2023Tool has the potential to assist with preterm birth labor management and clinical decision-making.“EMMI has the potential to answer critical questions about uterine contractions and will help us better understand what occurs during pregnancy and labor ” said Diana W. Bianchi M.D. NICHD Director. “With additional research the tool may potentially predict who is at risk to deliver prematurely or whose labor pattern will eventually result in the need for a cesarean section delivery. This will also help care providers evaluate whether a treatment or intervention is working.”The study team led by Yong Wang Ph.D. and Alan Schwartz M.D. Ph.D. at Washington University in St. Louis and Alison Cahill M.D. at the University of Texas at Austin initially developed EMMI using a sheep model and reported their findings in Science Translational Medicine. In the new study the team tailored EMMI for human clinical use and tested it among a group of 10 women with healthy pregnancies. Current clinical methods to measure contractions (i.e. tocodynamometry and an intrauterine pressure catheter) can only provide limited details such as contraction duration and intensity while also being invasive.EMMI integrated two types of non-invasive scans—a fast anatomical MRI to obtain an image of the uterus (which can be taken during early term pregnancy or 37 weeks gestation) and a multi-channel surface scanning electromyogram that uses sensors placed along the belly to measure contractions during labor. These data are then combined and processed into three-dimensional uterine maps with warm colors denoting areas of the uterus that are activated earlier in a contraction cool colors indicating areas that are activated later and gray areas showing inactive regions. A sequence of maps is generated over time creating a visual timelapse that shows where contractions start how they spread and/or synchronize and potential patterns that are associated with a typical pregnancy versus one with complications.EMMI maps were also used to develop metrics to describe uterine contractions. The maximal activation ratio for example measures the total surface area of the uterus that becomes electrically active during an individual contraction. The activation curve slope measures the rate of uterine electrical activation. The fundal early activation ratio helps quantify the region that generates contractions to dilate the cervix.Results from the pilot study also bring clarity on a longstanding question on how contractions begin—EMMI data suggest there is no fixed pacemaker-like region in the uterus that initiates labor. The study team observed varied patterns of contractions and metrics among the 10 study participants with some similarities between women who had never given birth and those who had. However more research is needed to confirm and expand upon these observations.EMMI offers new possibilities for better understanding human labor and facilitating the development of optimized patient-specific interventions. The authors note that an EMMI contraction atlas generated from healthy pregnancies can serve as a resource to understand and diagnose preterm labor and possibly identify patients who would benefit from an induction versus those who may need a cesarean section.Wang H. Wen Z. et. al. Noninvasive electromyometrial imaging of human uterine maturation during term labor. Nature Communications 10.1038/s41467-023-36440-0 (2023)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/new-non-invasive-imaging-tool-maps-uterine-contractions-during-labor
Visually navigating on foot uses unique brain region
Wednesday March 15 2023Occipital place area supports visually guided navigation but only when walking; not crawling.Navigating through a physical environment — anything from a small room to a city — requires the brain to process several classes of information. Each class of information is processed in its own region of the brains cortex which then work together to support navigation behavior such as walking. Loss of any one of these regions can affect how or whether someone can successfully navigate.Two main areas of the cortex are activated as people navigate through an environment the OPA and the retrosplenial complex (RSC). Daniel Dilks Ph.D. Emory University Atlanta theorizes that each of these areas supports a different kind of navigation. The RSC supports map-based navigation which involves finding our way from a specific place to some distant out-of-sight place (for example finding our way from your house to your favorite restaurant). By contrast he believes the OPA supports visually guided navigation which involves finding our way through near environment avoiding boundaries and obstacles (for example moving through your kitchen without bumping into things).However his theory has been controversial in part because the OPA doesnt appear to support visually guided navigation until around 8 years of age. Yet children somehow manage to get around their homes and schools long before that time – even from the earliest ages when they crawl rather than walk.“We asked ourselves does the OPA come on early but just mature slowly?” Dilks said. “Or does crawling use an entirely different system?”While most adults and older children primarily navigate environments by walking we retain the ability to crawl as we did in infancy. If OPA just matured slowly then it should be activated by both modes of movement Dilks reasoned. So he and students Christopher Jones and Joshua Byland set out to discover whether the OPA would activate in adults when crawling.To test this the scientists recorded videos from the perspective of someone walking through an environment and then similar videos from the perspective of someone crawling through that same environment. They also patched together random shots of the videos (scrambled) and took videos from a flying-over-the-environment perspective to include a mode of navigation not accessible to humans.When viewing videos our brains often activate as if we were performing the activity ourselves – a sympathetic response that made Dilks experiment possible. Using functional magnetic resonance imaging (fMRI) the researchers were able to monitor the activation of brain regions in 15 adult study participants as they were viewing each video and imagining themselves moving through the environment.When the participants viewed the walking video the region of the brain corresponding to the OPA was activated. But when they viewed the other videos – crawling flying or scrambled OPA was not activated. In contrast the RSC was activated when viewing all the videos suggesting that only OPA is specific for walking as opposed to other modes of visual navigation.In addition several other brain areas were activated when the participants viewed the crawling videos suggesting additional regions that may be involved in navigation early in life.“Not only does this study suggest that theres a completely different brain system managing navigation in early versus late childhood but it suggests that each of these pieces of the navigation system come on at different stages of development ” Dilks said. “Based on our study we think OPA is specifically tied to mature efficient walking.”The study was funded by the National Eye Institute (R01 EY29724).This press release describes a basic research finding. Basic research increases our understanding of human behavior and biology which is foundational to advancing new and better ways to prevent diagnose and treat disease. Science is an unpredictable and incremental process — each research advance builds on past discoveries often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research. To learn more about basic research visit https //www.nih.gov/news-events/basic-research-digital-media-kit.NEI leads the federal governments research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information visit https //www.nei.nih.gov.Jones CM Byland J and Dilks DD. “The occipital place area represents visual information about walking not crawling.” Cerebral Cortex March 15 2023. https //doi.org/10.1093/cercor/bhad055U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/visually-navigating-foot-uses-unique-brain-region

Scientists reveal a potential new approach to treating liver cancer
Monday March 13 2023Results in cell and mouse studies may have implications for the development of a new class of anticancer drugs.The researchers suggest that this enzyme could become a potential target for the development of new drugs against liver cancers and perhaps other cancers and diseases as well.Hall Nabeel Bardeesy Ph.D. a liver cancer specialist at Massachusetts General Hospital and their colleagues reported their results March 13 in Nature Cancer.The finding stems from a collaboration between Massachusetts General Hospital and NCATS researchers. Bardeesy was originally studying cholangiocarcinoma a type of liver cancer that affects the bile duct. The cancer is characterized by mutations in the IDH1 enzyme. Bardeesys team wanted to find compounds and drugs that might be effective against the IDH1 mutation. Through a collaboration with NCATS Hall and other NCATS scientists rapidly tested thousands of approved drugs and experimental cancer agents for their effectiveness in killing cholangiocarcinoma cells with IDH1 as a target.They found several molecules including one called YC-1 could kill the cancer cells. Yet when they looked to see how YC-1 was working they discovered the compound wasnt affecting the IDH1 mutation.The Massachusetts researchers showed that the liver cancer cells made an enzyme SULT1A1. The enzyme activated the YC-1 compound making it toxic to tumor cells in cancer cell cultures and mouse models of liver cancers. In the animal models treated with YC-1 the liver tumors either had reduced growth or shrank. Conversely the researchers found no changes in tumors treated with YC-1 in animals with cancer cells lacking the enzyme.The researchers examined other databases of drug screening results in compound and drug libraries to match drug activity with SULT1A1 activity. They also looked at a large National Cancer Institute database of anticancer compounds for additional possibilities to test for their activity with the enzyme.They identified several classes of compounds that relied on SULT1A1 for their tumor-killing activity. Using computational methods they predicted other compounds that also likely were dependent on SULT1A1.“Once we found SULT1A1 activated YC-1 it led us to ask What other compounds are active and can kill cells by the same mechanism? Hall said. “Can we identify other compounds that were being developed and demonstrate that they were also active because of SULT1A1 activation? The answer was yes. We found other compounds with the same mechanism of action as YC-1.”The scientists suggest these findings have broader implications for developing new anticancer drugs. “We think these molecules have the potential to be an untapped class of anticancer drugs that depend on SULT1A1 for their activity against tumors ” Bardeesy said.The researchers see YC-1 and similar molecules as prototypes for developing compounds that could be effective against important proteins on cells. Modifying different parts of these molecules could make them more specific for such proteins. The researchers point to the creation of a “toolkit of SULT1A1-activated molecules” that could affect many different targets.Such a toolkit is comprised of hundreds of known molecules. In theory the toolkit covers many types of enzymes called sulfotransferases that are active in different tissues in the body. For example in addition to SULT1A1 the human sulfotransferase SULT4A1 is active in the brain. It can activate a subset of the molecules in the toolkit. This might be useful in developing drugs specific for brain cancers.“We knew SULT1A1-dependent drugs had already been identified ” Bardeesy said. “Our results suggest there could be other SULT1A1-dependent compounds with ranges of different targets. Identifying such compounds and targets on cells could have potential implications for developing other types of small molecules and drugs not just limited to these cancers. This might become a new approach for some diseases.”This work was supported by the MGH Fund for Medical Discovery Award; the Cholangiocarcinoma Foundation Christopher J. Wilke Memorial Research Fellowship; NCI 1K99CA245194-01 the V Foundation for Cancer Research the Department of Defense Translational Team Science Award W81XWH-17-1-0491; NCI SPORE P50 CA127003; the Gallagher Chair in Gastrointestinal Cancer Research and Target Cancer Foundation; and the MGH Excellence Award.About the National Center for Advancing Translational Sciences (NCATS) NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about how NCATS helps shorten the journey from scientific observation to clinical intervention visit https //ncats.nih.gov.National Center for Advancing Translational Sciences (NCATS)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/scientists-reveal-potential-new-approach-treating-liver-cancer
Large-scale genetic analysis shows microRNAs in human pancreas associated with diabetes
Thursday February 9 2023In a new large-scale genetic analysis scientists have found a set of small RNA molecules called microRNAs in human pancreatic cells that are strongly associated with type 2 diabetes. Researchers discovered the microRNAs in groups of cells called pancreatic islets which produce hormones such as insulin that the body uses to regulate energy levels.In people with diabetes the islets fail to produce sufficient insulin to control blood sugar which is why understanding the basic biology of pancreatic islets is important for human health.Previous research with animal or cell-based models over the past two decades suggests that certain microRNAs which are involved in controlling which genes are turned on and off in cells may help pancreatic islets normally develop and function.However the specific role of these microRNAs in human pancreatic islets was poorly characterized.“This study represents the largest sequenced-based analysis of microRNA expression in human pancreatic islets to date ” said Francis Collins M.D. Ph.D. senior investigator in the Center for Precision Health Research in NHGRIs Intramural Research Program and senior author of the study. “The results of this study set the stage for understanding how microRNAs fine-tune gene expression in pancreatic islets and its implications for diabetes.”Using next-generation DNA sequencing a fast and high-throughput method for sequencing nucleic acids (like DNA and RNA) the researchers analyzed microRNAs in over 60 samples of human pancreatic islets. They found specific microRNAs that are different in people with type 2 diabetes which may be important for charting the course of the condition or for future development of drug therapies.“Some of the human pancreatic islet microRNAs we found to be associated with diabetes have not been well-studied previously ” said Praveen Sethupathy Ph.D. professor of physiological genomics at Cornell University Ithaca New York and senior author of the study. “The majority of prior studies have been in rodents or in islet-like groups of cells growing in a dish. It has not been clear whether or to what extent the discoveries made in these studies are relevant to human pancreatic islets and diabetes.”The researchers also found genomic variants that are associated with the quantity (or expression level) of certain microRNAs in the cell. These genomic variants might explain the variation seen in the level of specific microRNAs among different people. One of these genomic variants was found in a genomic region known to be associated with increased risk for type 2 diabetes-related traits which may give researchers clues about how type 2 diabetes develops.Diabetes affects more than 37 million Americans — about 1 in 10 people. Approximately 90-95% of people with diabetes have type 2 diabetes in which the body either does not make enough insulin and/or is unable to use insulin properly. The hallmark feature of diabetes is too much sugar circulating in the bloodstream. Prolonged high blood sugar levels can lead to heart disease kidney disease and vision loss. Currently diabetes has no cure but treatment and lifestyle changes can help individuals manage the condition.Taylor H. et al Human pancreatic islet microRNAs implicated in diabetes and related traits by large-scale genetic analysis. February 9 2023. PNAS. https //doi.org/10.1073/pnas.2206797120National Human Genome Research Institute (NHGRI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/large-scale-genetic-analysis-shows-micrornas-human-pancreas-associated-diabetes

Early anti-VEGF treatment of diabetic retinopathy yields no benefit to visual acuity
Tuesday February 7 2023“This study indicates that monitoring patients regularly for vision-threatening diabetes complications and treating eyes only as needed is the best approach ” said Raj Maturi M.D. Indiana University School of Medicine and Retina Partners Midwest who is the protocol chair for the four-year study. The report was published today in the Journal of the American Medical Association (JAMA).Anti-vascular endothelial growth factor (anti-VEGF) drugs can substantially decrease the risk of vision loss from diabetic retinopathy. However eye care providers have been unsure when treatment should start to achieve the best long-term outcome. Anti-VEGF is given by injection into the eye so physicians must weigh the risks for side effects and the expense and inconvenience of treatment against potential treatment benefit.Diabetic retinopathy occurs when diabetes affects the blood vessels of the eyes light-sensing retina. In early stages diabetes weakens retinal blood vessels causing fluid to leak into the surrounding retina. This stage is called non-proliferative diabetic retinopathy (NPDR). Fluid buildup in the retina called diabetic macular edema is a complication of diabetic retinopathy and can lead to vision loss. Progression of the disease to proliferative diabetic retinopathy (PDR) where new abnormal blood vessels begin to grow in the retina can also threaten vision.NEI-funded researchers evaluated whether treating people with NPDR with the anti-VEGF drug Eylea (aflibercept) could prevent vision loss. The study enrolled 328 participants with 399 study eyes (some participants had two eyes that met criteria for enrollment in the study; others only had one study-eligible eye). Preventive anti-VEGF injections were given in 200 eyes at one month after enrollment two months and four months and then every four months over two years. Preventative treatment continued every four months through four years unless NPDR improved to only mild disease. Sham injections (without drug) were used in 199 eyes over the same period. Any eye that developed a vision-threatening complication such as macular edema or PDR was treated with additional anti-VEGF injections as necessary.Two-year results of the study suggested that while preventive treatment reduced the risk of developing diabetic macular edema or PDR there was no evident benefit to vision. These final four-year results reinforce the earlier finding with no statistical difference in either visual acuity or rates of vision loss between the two groups.“We expected early treatment to prevent progression of diabetic retinopathy but even with preventative injections about one-third of eyes developed vision-threatening complications ” said Adam Glassman Jaeb Center for Health Research Tampa Florida who directs the DRCR Retina Network coordinating center.Over the four-year study 34% percent of eyes receiving preventive treatments showed disease progression compared with 57% of those in the sham group. On average those in the preventive group received 11 injections compared with an average of three in the sham group.“While the individual risk of complications per injection is low the risk increases with each additional injection ” said Jennifer Sun M.D. M.P.H. Joslin Diabetes Center Harvard Medical School Boston and chair of diabetes initiatives for the DRCR Retina Network. “The results of this study indicate that the anatomic benefit from early anti-VEGF treatment does not result in improved visual acuity and so it may not be worth the risk and inconvenience to the patient of repeat preventive injections for NPDR.”NEI leads the federal governments research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information visit https //www.nei.nih.gov.Maturi RK Glassman AR Josic K Baker CW Gerstenblith AT Jampol LM Meleth A Martin DF Melia M Punjabi OS Rofagha S Salehi-Had H Stockdale CR and Sun JK for the DRCR Retina Network. “Four-Year Visual Outcomes in a Randomized Trial of Intravitreous Aflibercept for Prevention of Vision Threatening Complications of Diabetic Retinopathy (Protocol W).” JAMA. February 7 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/early-anti-vegf-treatment-diabetic-retinopathy-yields-no-benefit-visual-acuity
Low-dose aspirin may increase risk of anemia in older adults
Tuesday June 20 2023Published in the Annals of Internal Medicine scientists from the Aspirin in Reducing Events in the Elderly (ASPREE) study examined the effect of long-term low-dose aspirin use on incident anemia and the effect of aspirin on changes in hemoglobin concentration as well as ferritin levels as an indicator of iron deficiency. The researchers found that low-dose aspirin led to increased incident anemia in otherwise healthy older adults at enrollment independent of major bleeding.Previous ASPREE data analyses suggested daily low-dose aspirin does not decrease risk for dementia and cognitive decline; and that daily low-dose aspirin had no effect on healthy lifespan in older people.ASPREE a joint U.S. and Australian research project aimed at determining the effect of low-dose aspirin on survival without dementia or disability began in 2010 and completed recruitment in 2014. It was a randomized double-blind placebo-controlled primary prevention trial of daily 100 mg of aspirin in a population of healthy older people in the U.S. and Australia with a period of treatment averaging 4.5 years. The trial involving 19 114 people age 65 and older was distinctive for its size methodological rigor and high participant retention rate in both countries.The following NIA experts are available to discuss specific findings of this paperMcQuilten ZK et al. Effect of Low Dose Aspirin versus Placebo on Incidence of Anemia in the Elderly An Analysis of the Aspirin in Reducing Events (ASPREE) Randomized Controlled Trial. Annals of Internal Medicine. 2023; doi 10.7326/M23-0675U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/low-dose-aspirin-may-increase-risk-anemia-older-adults

Lingering symptoms common after COVID hospitalization
Tuesday February 14 2023Many adults experience problems like coughing chest pain and fatigue six months after their stay.After six months more than 7 in 10 adults surveyed in the study experienced cardiopulmonary problems such as coughing rapid or irregular heartbeat and breathlessness while about half had fatigue or physical limitations – all symptoms associated with long COVID. Additionally more than half of the adults said they faced financial challenges.“My clinic patients often want to know how soon theyll get back to their usual health ” said Andrew J. Admon M.D. M.P.H. the studys first author a pulmonologist at LTC Charles S. Kettles VA Medical Center and an assistant professor in the departments of internal medicine and epidemiology at the University of Michigan Ann Arbor. “Based on these data it seems that many people hospitalized for COVID-19 should expect symptoms to last for up to six months or even longer.”To conduct the analysis researchers assessed data from the medical records and follow-up surveys of 825 adults who received treatment for COVID at one of 44 medical centers in the United States between August 2020 and July 2021. Patients were surveyed one three and six months after leaving the hospital for general or intensive care treatment.The researchers found that six months after being hospitalized“These findings will inform programs designed to help adults recover from severe cases of COVID and guide how physicians should check in with patients in the year following hospitalization ” said James P. Kiley Ph.D. the director of NHLBIs Division of Lung Diseases. “They may also help shape future clinical research studies.”For a bigger picture other studies such as the Household Pulse Survey have suggested that half of U.S. adults have reported having COVID. International data suggest that about 1 in 13 adults 6-7% who have had symptomatic COVID infections still experienced symptoms months later. Based on research published in JAMA symptoms lasted for about four months for those who recovered outside of the hospital compared to nine months for those who received hospital care.To learn more about BLUE CORAL visit https //petalnet.org/studies/public/bluecoral.About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit https //www.nhlbi.nih.gov/.Admon AJ Iwashyna TJ Kamphuis LA et al. Assessment of symptom disability and financial trajectories in patients hospitalized for COVID-19 at 6 months. JAMA Network Open. 2023; doi 10.1001/jamanetworkopen.2022.55795.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/lingering-symptoms-common-after-covid-hospitalization

Study finds spinal cord stimulation may restore arm and hand mobility after stroke
Tuesday February 21 2023The technology uses a set of thin metal electrodes implanted on the surface of the spinal cord. Electrical impulses from the device stimulate neural circuits in the spinal cord priming them to receive movement signals from the brain. This engages muscles that have been weakened by stroke allowing patients to voluntarily lift their arm open and close their fist and grasp household objects.Recent studies have used spinal cord stimulation technology to treat chronic pain and restore leg movement after spinal cord injury. Building on years of extensive preclinical studies using computer modeling and non-human primates and a pilot study in humans researchers tested the new therapy in two stroke patients with moderate to severe motor impairments.They found that continuous stimulation targeting the cervical sensory nerve roots of the spinal cord immediately improved strength range of motion and function of the arm and hand. Stimulation also enabled participants to perform complex tasks that require more skill and dexterity such as using utensils to eat and opening a lock activities that they had not done in years.Surprisingly some benefits persisted for several weeks after the device was removed. This suggests that when combined with physical or occupational therapy this assistive stimulation approach could lead to more robust long-term improvements in motor function.Globally one in four people over age 25 will suffer from a stroke and nearly three-quarters of these individuals will have lasting deficits in the motor control of their arm and hand causing disability and enormous impacts on daily life. There are no effective treatments for paralysis in the chronic stage of stroke which begins six months after the initial stroke incident.These findings provide a practical stimulation protocol for adapting an existing clinical technology to treat upper-limb paralysis following stroke. More research is needed to translate the therapy into broader clinical use. Future studies will examine ways to further optimize stimulation protocols and determine which stroke patients can benefit most from the therapy.study findings. To arrange an interview please contact NINDSPressTeam@ninds.nih.gov.Powell M.P. et al. Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Nature Medicine. Feb 20 2023. DOI 10.1038/s41591-022-02202-6.NINDS is the nations leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/study-finds-spinal-cord-stimulation-may-restore-arm-hand-mobility-after-stroke

Tranexamic acid does not appear to prevent maternal hemorrhage after cesarean delivery
Thursday April 13 2023In addition patients who received tranexamic acid had slightly less need for additional medical or surgical interventions to treat postpartum hemorrhage and a slightly lower drop in red blood cell count.“Our findings differ from those of previous trials which were smaller and therefore did not have the statistical power to detect a difference in the need for blood transfusion between groups ” said study author Monica Longo M.D. of the NICHD Pregnancy and Perinatology Branch. “The current study included participants from 31 birthing centers across the United States and found no benefit of the drug compared to placebo.”Previously researchers have theorized that since tranexamic acid prevents the breakdown of blood clots the drug might slow blood loss and reduce the risk of postpartum hemorrhage. Tranexamic acid has been found to be effective among women experiencing postpartum hemorrhage. Researchers sought to determine the effectiveness of the drug for patients undergoing cesarean delivery who did not have hemorrhaging at the time of treatment.Researchers assigned 11 000 patients to receive either intravenous tranexamic acid or placebo after umbilical cord clamping at the time of cesarean delivery. The study included women who had undergone scheduled and unscheduled cesarean delivery.The researchers reported the results as a single primary outcome of events that might be expected with postpartum hemorrhage the need for a transfusion of red blood cells or death. These events occurred in 201 patients (3.6%) in the tranexamic acid group and 233 (4.3%) in the placebo group a difference that was not statistically significant. For the placebo group one death occurred. There were no deaths in the tranexamic acid group. There was no significant difference between the groups for the secondary outcome of estimated blood loss of more than 1 liter during the procedure 7.3% in the tranexamic acid group 8% in the placebo group.However the study found that patients who received tranexamic acid had less need for additional medical or surgical interventions to treat postpartum hemorrhage compared to the placebo group (16.1% versus 18%) and a lower drop in red blood cell count after cesarean delivery (1.8 grams per deciliter versus 1.9 grams per deciliter).The tranexamic acid group was more likely to have an infection after giving birth 3.2% to 2.5%. The authors noted that previous trials had not seen a difference in infection rates between the groups and added that the finding would need to be confirmed by additional research.Pacheco LD et al. Tranexamic acid to prevent obstetrical hemorrhage after cesarean delivery. The New England Journal of Medicine. 2023.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/tranexamic-acid-does-not-appear-prevent-maternal-hemorrhage-after-cesarean-delivery

Increased use of telehealth services and medications for opioid use disorder during the COVID-19 pandemic associated with reduced risk for fatal overdose
Wednesday March 29 2023Study findings support the continuation of providing telehealth services for opioid use disorder and its related care.The expanded availability of opioid use disorder-related telehealth services and medications during the COVID-19 pandemic was associated with a lowered likelihood of fatal drug overdose among Medicare beneficiaries according to a new study.“The results of this study add to the growing research documenting the benefits of expanding the use of telehealth services for people with opioid use disorder as well as the need to improve retention and access to medication treatment for opioid use disorder ” said lead author Christopher M. Jones PharmD DrPH director of the National Center for Injury Prevention and Control Centers for Disease Control and Prevention. “The findings from this collaborative study also highlight the importance of working across agencies to identify successful strategies to address and get ahead of the constantly evolving overdose crisis.”“CMS is committed to ensuring that the beneficiaries we serve can access the high-quality behavioral health services they need ” said senior author Dr. Shari Ling M.D. deputy chief medical officer at CMS. “This study shows that many beneficiaries were able to utilize opioid use disorder-related telehealth services during the pandemic but we need to continue our efforts to broaden the use of telehealth particularly in underserved communities.”In this national study researchers analyzed data among two cohorts of Medicare beneficiaries to explore receipt of opioid use disorder-related telehealth services receipt of medications for opioid use disorder and fatal overdoses before and during the COVID-19 pandemic. To do this they compared data from two cohorts of Medicare beneficiaries across two time periods. The first cohort was constructed with data from September 2018-February 2020 and included 105 162 Medicare beneficiaries with opioid use disorder (the “pre-pandemic cohort”). The second cohort was constructed with data from September 2019-February 2021 and included 70 479 Medicare beneficiaries with opioid use disorder (the “pandemic” cohort). In addition the researchers conducted an analysis to examine the demographic and clinical characteristics associated with fatal overdose in the pandemic cohort.Key findings of this study includeAlthough the results of this study were able to identify the positive impact opioid use disorder-related telehealth services had on lowering the risk for fatal drug overdose in the pandemic cohort the authors note that only 1 in 5 Medicare beneficiaries in the pandemic cohort received OUD-related telehealth services. Similarly only 1 in 8 beneficiaries in the pandemic cohort received medications for opioid use disorder. These findings underscore the need for continued expansion of these potentially life-saving interventions across clinical settings.Find Treatment for Substance Use Disorder including Opioid Use DisorderIf you or someone close to you needs help for a substance use disorder talk to your doctor or call SAMHSAs National Helpline at 1-800-662-HELP or go to SAMHSAs Behavioral Health Treatment ServicesIf you have questions about any medicines call the U.S. Department of Health and Human Services Poison Help Hotline at 1-800-222-1222.About CDC CDC works 24/7 protecting Americas health safety and security. Whether disease start at home or abroad are curable or preventable chronic or acute or from human activity or deliberate attack CDC responds to Americas most pressing health threats. CDC is headquartered in Atlanta and has experts located throughout the United States and the world.About the Centers for Medicare & Medicaid Services (CMS) CMS provides health coverage to nearly 150 million people through Medicare Medicaid the Childrens Health Insurance Program and the Health Insurance Marketplace. A component of the U.S. Department of Health and Human Services CMS serves the public as a trusted partner and steward dedicated to advancing health equity expanding coverage and improving health outcomes.Jones CM Shoff C Blanco C Losby JL Ling SM Compton WM. Association of Receipt of Opioid Use Disorder–Related Telehealth Services and Medications for Opioid Use Disorder With Fatal Drug Overdoses Among Medicare Beneficiaries Before and During the COVID-19 Pandemic. JAMA Psychiatry. March 29 2023. doi 10.1001/jamapsychiatry.2023.0310Centers for Medicare & Medicaid Services (CMS)Centers for Disease Control and PreventionU.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/increased-use-telehealth-services-medications-opioid-use-disorder-during-covid-19-pandemic-associated-reduced-risk-fatal-overdose

National Science Advisory Board for Biosecurity to meet
Tuesday January 17 2023Meeting will take place on Jan. 27 2023.The National Science Advisory Board for Biosecurity (NSABB) will convene virtually on January 27 2023 to discuss the draft report of the NSABB Working Groups to Review and Evaluate the U.S. Government Potential Pandemic Pathogen Care and Oversight (PC3O) and Dual Use Research of Concern (DURC) Policies. The draft report will be posted on the NSABB website ahead of the meeting. Members of the public may provide comments on the draft report at the meeting or submit comments in writing.Friday January 27 2023 1-3 p.m. ETWebcast Information https //videocast.nih.govInformation on how to participateU.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/national-science-advisory-board-biosecurity-meet-1
Researchers identify compounds that could lead to an on-demand, short-term contraceptive for men
Tuesday February 14 2023In several tests the researchers showed that the compound TDI-11861 rendered mouse sperm cells immobile and prevented them from maturing. The compounds did not interfere with the animals sexual functioning. Although male mice mated with females no pregnancies were observed. Sperm recovered from female mice remained incapacitated. The authors did not observe any side effects in the male or female mice. The compound wore off three hours later and males recovered their fertility.The researchers say their work provides proof of concept that soluble adenylyl cyclase inhibitors have the potential to provide a safe on-demand non-hormonal and reversible oral contraceptive for men.Christopher C. Lindsey Ph.D. a program official in the NICHD Contraception Research Branch is available for comment.Balbach M. Research Letter On-demand male contraception via acute inhibition of soluble adenylyl cyclase. Nature Communications.2022. https //doi.org/10.1038/s41467-023-36119-6U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/researchers-identify-compounds-could-lead-demand-short-term-contraceptive-men

Biden-Harris Administration launches initiative to improve cancer outcomes in low-income areas
Monday June 26 2023Persistent poverty areas are defined as those where for the past 30 years 20% or more of the population has lived below the federal poverty line. People who live in such areas have a higher incidence of cancer experience delays in cancer diagnosis and treatment and are more likely to die from cancer than people who do not live in poverty. However there has been limited research on how to improve cancer outcomes in persistent poverty areas.“Persistent poverty is a place-based and community phenomenon that reflects a failure of the structures and institutions in society including health care ” said Shobha Srinivasan Ph.D. senior advisor for health disparities and health equity in NCIs Division of Cancer Control and Population Sciences. “Conducting research to understand the connections between institutions — such as social economic and health systems — and persistent poverty is the only way to inform changes to social conditions and determinants of health that will ultimately improve overall health cancer control and cancer outcomes.”Each center will work with targeted low-income communities to implement and measure the effectiveness of structural interventions for cancer control and prevention follow-up care and survivorship. These centers will conduct research in areas such as reducing obesity improving nutrition increasing physical activity helping people quit smoking and improving living conditions through supplemental income. In addition the centers will help train a pipeline of early-career investigators to work with underserved communities in conducting multilevel intervention research.“By involving the community and making the community an essential part of this effort we are building a sustainable model ” Dr. Srinivasan said. “The idea of structural change then becomes much more built into the system.”The launch of this Persistent Poverty Initiative complements several other Administration priorities including its efforts to end hunger and reduce diet-related disease and is essential toward achieving the clear goals that President Biden and the First Lady set when they reignited the Cancer Moonshot in 2022 prevent more than 4 million cancer deaths by 2047 and improve the experience of people who are touched by cancer.The awards are spread over the next five years across all centers pending availability of fundsFor more information about NCIs investments in underserved areas visit https //cancercontrol.cancer.gov/hdhe/research-emphasis/underserved-areasU.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/biden-harris-administration-launches-initiative-improve-cancer-outcomes-low-income-areas
New 3-D model offers insights into the role of glucose in a deadly kidney disease
Thursday January 5 2023In PKD tiny tubes (tubules) in the kidneys expand like water balloons forming sacs of fluid over decades. The sacs or cysts eventually crowd out healthy tissue leading to problems in kidney function and kidney failure. Scientists have identified many of the genes that cause PKD but much about the disease remains unknown including how the cysts form.“Were able to boil down a complex process of cyst formation in tubules into a process in a petri dish that takes just a few weeks but theres been a lack of technologies to study the disease further ” said University of Washington School of Medicine scientist Benjamin Freedman Ph.D. who led the work. “Animal models are helpful but translating the results of those studies to peopl has been a challenge.”Freedman co-author Jonathan Himmelfarb M.D. and their Seattle-based colleagues decided to explore combining organoid technology with a tissue-chip platform. Scientists believe that fluid flow is important in the development of cysts but they had no way of testing the theory in organoids.“In kidneys fluid is always going through the tubules; at any given moment the kidneys have about 25% of the body fluid going through them ” Freedman explained. “We cant reproduce this system in the dish because fluid needs to move through the kidney structures. Using microfluidic technology in tissue chips was a natural next step.”Freedmans group showed that exposing the PKD organoid-on-a-chip model to a combination of water sugar amino acids and other nutrients caused cysts to expand relatively quickly. They found that the cysts were absorbing glucose and pulling in water from the fluid passing over them making the cysts grow larger. Although glucose is generally absorbed by the kidneys glucose absorption has not been connected to cyst formation in PKD.“It wasnt a huge surprise that the cysts could absorb glucose but it was surprising that they were dependent on it. Its a new way of thinking of how these cysts form ” Freedman said.The scientists added fluorescent glucose to mice with PKD and found that the mouse cysts also took up the glucose. “We think the tubules are taking in fluid in the mice just like in the organoids. The kidney gets bigger and as the tubules widen to accommodate the expansion over time cysts form ” Freedman said.Understanding the mechanisms of PKD can point to new ways to treat it. As part of the study the research team showed that adding compounds that block glucose transport prevented cyst growth. Freedman noted glucose inhibitors are being developed for other types of kidney disease.“The researchers have shown that simulating fluid flow is essential to making this system more like the environment in the kidney with PKD ” said Danilo Tagle Ph.D. director of the NCATS Office of Special Initiatives. “Combining the two technologies makes tissue chip technology more adaptable to drug discovery and drug development and allows researchers to take advantage of the strengths of both platforms. This is very promising for studying other diseases in new ways in the future.”About the National Center for Advancing Translational Sciences (NCATS) NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about how NCATS helps shorten the journey from scientific observation to clinical intervention visit https //ncats.nih.gov.National Center for Advancing Translational Sciences (NCATS)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/new-3-d-model-offers-insights-into-role-glucose-deadly-kidney-disease

SARS-CoV-2 infection weakens immune-cell response to vaccination
Monday March 20 2023The magnitude and quality of a key immune cells response to vaccination with two doses of the Pfizer-BioNTech COVID-19 vaccine were considerably lower in people with prior SARS-CoV-2 infection compared to people without prior infection a study has found. In addition the level of this key immune cell that targets the SARS-CoV-2 spike protein was substantially lower in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Importantly people who recover from SARS-CoV-2 infection and then get vaccinated are more protected than people who are unvaccinated. These findings which suggest that the virus damages an important immune-cell response were published today in the journal Immunity.Dr. Davis and colleagues designed a very sensitive tool to analyze how immune cells called CD4+ T cells and CD8+ T cells respond to SARS-CoV-2 infection and vaccination. These cells coordinate the immune systems response to the virus and kill other cells that have been infected helping prevent COVID-19. The tool was designed to identify T cells that target any of dozens of specific regions on the viruss spike protein as well as some other viral regions. The Pfizer-BioNTech vaccine uses parts of the SARS-CoV-2 spike protein to elicit an immune response without causing infection.The investigators studied CD4+ and CD8+ T-cell responses in blood samples from three groups of volunteers. One group had never been infected with SARS-CoV-2 and received two doses of the Pfizer-BioNTech COVID-19 vaccine. The second group had previously been infected with SARS-CoV-2 and received two doses of the vaccine. The third group had COVID-19 and was unvaccinated.The researchers found that vaccination of people who had never been infected with SARS-CoV-2 induced robust CD4+ and CD8+ T-cell responses to the virus spike protein. In addition these T cells produced multiple types of cell-signaling molecules called cytokines which recruit other immune cells—including antibody-producing B cells—to fight pathogens. However people who had been infected with SARS-CoV-2 prior to vaccination produced spike-specific CD8+ T cells at considerably lower levels—and with less functionality—than vaccinated people who had never been infected. Moreover the researchers observed substantially lower levels of spike-specific CD8+ T cells in unvaccinated people with COVID-19 than in vaccinated people who had never been infected.Taken together the investigators write these findings suggest that SARS-CoV-2 infection damages the CD8+ T cell response an effect akin to that observed in earlier studies showing long-term damage to the immune system after infection with viruses such as hepatitis C or HIV. The new findings highlight the need to develop vaccination strategies to specifically boost antiviral CD8+ T cell responses in people previously infected with SARS-CoV-2 the researchers conclude.F Gao et al. Robust T cell responses to the Pfizer/BioNTech vaccine compared to infection and evidence of attenuated CD8+ T cell responses due to COVID-19. Immunity DOI 10.1016/j.immuni.2023.03.005. (2023).Dan Rotrosen M.D. director NIAID Division of Allergy Immunology and Transplantation is available to respond to media requests for interviews.To schedule interviews please contact Laura S. Leifman (301) 402-1663 NIAIDNews@niaid.nih.gov.U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/sars-cov-2-infection-weakens-immune-cell-response-vaccination

Omega-3 fatty acids appear promising for maintaining lung health
Thursday July 20 2023“We know a lot about the role of diet in cancer and cardiovascular diseases but the role of diet in chronic lung disease is somewhat understudied ” said corresponding author Patricia A. Cassano Ph.D. director of the Division of Nutritional Sciences at Cornell University in Ithaca New York. “This study adds to growing evidence that omega-3 fatty acids which are part of a healthy diet may be important for lung health too.”Theres increased interest in trying to understand whether nutritional interventions could contribute to lung disease prevention efforts. Past studies have suggested that omega-3 fatty acids may help due largely to their established anti-inflammatory actions. However robust studies of this association have been lacking until now.The participants studied were generally healthy when the study began and the majority had no evidence of chronic lung disease. They comprised a racially diverse group of adults with an average age of 56 years and 55% were female. The researchers followed participants for an average of seven years and up to 20 years. The longitudinal study showed that higher levels of omega-3 fatty acids in a persons blood were associated with a reduced rate of lung function decline. The researchers observed the strongest associations for docosahexaenoic acid (DHA) an omega-3 fatty acid that is found at high levels in fatty fish such as salmon tuna and sardines. DHA is also available as a dietary supplement.In the second part the researchers analyzed genetic data from a large study of European patients (over 500 000 participants) from the UK Biobank. They studied certain genetic markers in the blood as an indirect measure or proxy for dietary omega-3 fatty acid levels to see how they correlated with lung health. The results showed that higher levels of omega-3 fatty acids — including DHA — were associated with better lung function.One caveat of the current study is that it only included healthy adults. As part of this ongoing project researchers are collaborating with the COPDGene study to examine blood levels of omega-3 fatty acids in relation to the rate of decline in lung function among people with chronic obstructive pulmonary disease or COPD — including heavy smokers — to determine if the same beneficial associations are found.“Were starting to turn a corner in nutritional research and really moving toward precision nutrition for treating lung diseases ” said study first author Bonnie K. Patchen Ph.D. a nutritionist and member of Cassanos research team at Cornell. “In the future this could translate into individualized dietary recommendations for people at high risk for chronic lung disease.”For now the researchers point out that the U.S. Department of Agricultures Dietary Guidelines for Americans recommends that people eat at least two servings of fish per week which most Americans fall far short. In addition to fish and fish oil other sources of omega-3 fatty acids include nuts and seeds plant oils and fortified foods.“This large population-based study suggests that nutrients with anti-inflammatory properties may help to maintain lung health ” said James P. Kiley Ph.D. director of the NHLBIs Division of Lung Diseases. “More research is needed since these findings raise interesting questions for future prospective studies about the link between omega-3 fatty acids and lung function.”About the National Heart Lung and Blood Institute (NHLBI) NHLBI is the global leader in conducting and supporting research in heart lung and blood diseases and sleep disorders that advances scientific knowledge improves public health and saves lives. For more information visit www.nhlbi.nih.gov.Investigating associations of omega-3 fatty acids lung function decline and airway obstruction. Am J Respir Crit Care Med. 2023; doi 10.1164/rccm.202301-0074OC.National Heart Lung and Blood Institute (NHLBI)U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/omega-3-fatty-acids-appear-promising-maintaining-lung-health

School prevalence of stimulant therapy for ADHD associated with higher rates of prescription stimulant misuse among teens
Tuesday April 18 2023Researchers have identified a strong association between prevalence of prescription stimulant therapy for attention-deficit/hyperactivity disorder (ADHD) and rates of prescription stimulant misuse (taken in a way other than as directed by a clinician) by students in middle and high schools. The study which appeared today in JAMA Network Open highlights the need for assessments and education in schools and communities to prevent medication-sharing among teens. This is especially important considering non-medical use of prescription stimulants among teens remains more prevalent than misuse of any other prescription drug including opioids and benzodiazepines.“The drug supply has rapidly changed and what looks like medications – bought online or shared among friends or family members – can contain fentanyl or other potent illicit substances that can result in overdoses. Its important to raise awareness of these new risks for teens ” said NIDA Director Nora Volkow M.D. “Its also essential to provide the necessary resources and education to prevent misuse and support teens during this critical period in their lives when they encounter unique experiences and new stressors.”Stimulant therapy is an evidence-based treatment for ADHD but it can also be harmful if used without prescription or guidance from clinicians. Prolonged stimulant misuse can lead to several detrimental health effects including cardiovascular conditions depressed mood overdoses psychosis anxiety seizures and stimulant use disorder.Previous studies have shown that more than half of adolescents who misuse prescription stimulants get the medication for free from friends or relatives. While diagnoses of ADHD and prescribing of stimulant therapy for ADHD have increased significantly in the United States over the past 20 years few studies have looked at the relationship between stimulant therapy and prescription stimulant misuse in schools. This is the first large national study to examine prevalence of prescription stimulant misuse and factors correlating with prevalence among students in eighth 10th and 12th grade across the U.S.Researchers at the University of Michigan examined both school- and individual-level characteristics associated with prescription stimulant misuse. Across 231 141 student participants surveyed at 3 284 secondary schools the school-level prevalence of nonmedical use varied from 0% to over 25% of students. Schools with a greater number of students (12% or higher) reporting prescription stimulant therapy for ADHD tended to have the highest percentages of their student body reporting prescription stimulant misuse (8% of total student body). By comparison schools with fewer students (0 to 6% of student body) reporting stimulant therapy for ADHD were associated with lower rates of prescription stimulant misuse (4 to 5% of student body).Other features of schools that were associated with increased rates of misuse included having a higher proportion of parents with higher levels of education being located in non-Northeastern regions and in suburban areas having a higher proportion of non-Hispanic white students and showing “medium-level” (10-19% of total student body) binge drinking. However the association between school prevalence of stimulant therapy for ADHD and prescription stimulant misuse remained strong when accounting for prevalence of other types of substance use and numerous other individual- and school-level sociodemographics.Recent research from this team expands on the associations found in this study including a study that suggested teens with a history of taking both stimulant or non-stimulant medications for ADHD are at high risk for prescription stimulant misuse as well as cocaine and methamphetamine use. The researchers note that it is important to interpret these results as associations not causations and that the primary goal of these kinds of studies is to inform effective preventative and support strategies for teens.“The key takeaway here is not that we need to lessen prescribing of stimulants for students who need them but that we need better ways to store monitor and screen for stimulant access and use among youth to prevent misuse ” said study author Sean Esteban McCabe Ph.D. “Theres variation in stimulant misuse across different schools so its important to assess schools and implement personalized interventions that work best for each school. Its also critical to treat and educate teens on prescription stimulants as the medications they are intended to be and limit their availability as drugs of misuse.”With sadness NIDA shares that study author and NIDA grantee Dr. John E. Schulenberg passed away in February. Dr. Schulenberg was a member of the Monitoring the Future studys leadership team for 32 years and has made immense contributions to addiction science that will be remembered and honored.For more information on substance and mental health treatment programs in your area call the free and confidential National Helpline 1-800-662-HELP (4357) or visit www.FindTreatment.gov.SE McCabe et al. Prescription stimulant medical and nonmedical use among US secondary school students (2005–2020). JAMA Network Open. DOI 10.1001/jamanetworkopen.20238707 (2023).U.S. Department of Health and Human Services
https://www.nih.gov/news-events/news-releases/school-prevalence-stimulant-therapy-adhd-associated-higher-rates-prescription-stimulant-misuse-among-teens